DOI:
10.1039/C8RA10532D
(Paper)
RSC Adv., 2019,
9, 8515-8528
Synthesis, anti-mycobacterial and cytotoxic evaluation of substituted isoindoline-1,3-dione-4-aminoquinolines coupled via alkyl/amide linkers†
Received
24th December 2018
, Accepted 3rd March 2019
First published on 13th March 2019
Abstract
A series of secondary amine-substituted isoindoline-1,3-dione-4-aminoquinolines were prepared via microwave heating and assayed for their anti-mycobacterial activities. The compound with a butyl chain as a spacer between the two pharmacophores and piperidine as the secondary amine component on the isoindoline ring was the most potent and non-cytotoxic among the synthesized compounds, exhibiting a minimum inhibitory concentration (MIC99) of 6.25 μg mL−1 against Mycobacterium tuberculosis.
Introduction
Tuberculosis (TB) is a global pandemic caused by the single infectious agent Mycobacterium tuberculosis. TB has been a scourge to mankind for thousands of years and roughly more than one third of the world's population is latently infected.1 In 2016, there were 10.4 million new cases of TB diagnosed with approximate mortality of 1.3 million among HIV-negative and an additional 374![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths among HIV-positive people, mostly in developing countries.2 According to the Revised National Tuberculosis Control Program (RNTCP), India accounts for one fourth of the global TB burden with 1.75 million TB patients, including data from both the public and private health sectors, while 33
000 deaths among HIV-positive people, mostly in developing countries.2 According to the Revised National Tuberculosis Control Program (RNTCP), India accounts for one fourth of the global TB burden with 1.75 million TB patients, including data from both the public and private health sectors, while 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 drug-resistant TB patients are noted additionally.3 Increasing cases of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB all over the world is hindering current TB treatment,4 which includes the first-line drugs isoniazid (INH), rifampicin (RIF), pyrazinamide, ethambutol, and streptomycin, and the second line drugs, aminoglycosides, polypeptides, fluoroquinolones, thioamides, cycloserine and terizidone.5,6 The emergence of resistant strains warrants the urgent search for faster-acting, less harmful, more efficient drug candidates.
820 drug-resistant TB patients are noted additionally.3 Increasing cases of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB all over the world is hindering current TB treatment,4 which includes the first-line drugs isoniazid (INH), rifampicin (RIF), pyrazinamide, ethambutol, and streptomycin, and the second line drugs, aminoglycosides, polypeptides, fluoroquinolones, thioamides, cycloserine and terizidone.5,6 The emergence of resistant strains warrants the urgent search for faster-acting, less harmful, more efficient drug candidates.
The quinoline core has received recent attention as it is one of the active key building blocks of many drugs. Bedaquiline – a diarylquinoline – was recently approved by Food and Drug Administration (FDA) and European Medicine Agency (EMA) for treating MDR-TB patients.7–9 However, the drug comes with a black-box warning, which includes the risk of cardiac (QT prolongation) and hepatic dysfunction and heightened risk of mortality.10 Mefloquine, an orally administered drug, is another quinoline derivative known for potent anti-tubercular activity.11–15 Remarkably, numerous derivatives of mefloquine were reported to have good antibacterial and anti-mycobacterial activities.16,17
Isoindoline-1,3-dione and its derivatives were shown to possess interesting biological properties18 against a wide panel of diseases and conditions, such as angiogenic disease,19 inflammation,20 cancer,21,22 Parkinson's,23 leprosy, AIDS and multiple myeloma (MM); as COX inhibitors; antidepressants and histone deacetylase inhibitors.24 Synthesis of conjugates of the isoindoline-1,3-dione subunit with different active moieties afforded active antimicrobials against Mycobacterium leprae and M. tuberculosis.25
A recent report from our lab highlighted the anti-mycobacterial potential of isoindoline-1,3-dione-4-aminoquinolines, exhibiting comparable activity with ethionamide and cephalexin (Fig. 1).26 In continuation of our efforts,27 we present herein the synthesis and anti-mycobacterial activities of secondary amino-substituted isoindoline-1,3-dione-4-aminoquinolines. The spacer length between two pharmacophores as well as the secondary amine functionality at the C4/C5 position of isoindoline-1,3-dione was meticulously altered for analysing the structure–activity relationship (SAR).
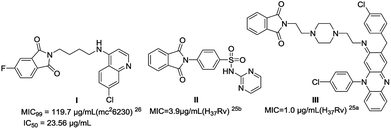 |
| | Fig. 1 Isoindoline-1,3-dione based active anti-TB compounds. | |
Results and discussion
Synthetic chemistry
For the synthesis of the desired isoindoline-1,3-dione-4-aminoquinolines with the secondary amine functionality at the C-4/C-5 position, microwave heating was attempted, as the reactions were sluggish when carried out under conventional heating. The microwave assisted reaction between 3-fluoro-phthalic anhydride 1a, N-(7-chloroquinolin-4-yl)diamine 2a28 and piperidine was chosen as model reaction. Different solvents viz. DMF, DMSO and NMP were screened, both for the single-pot as well as for the step-wise synthesis and the results are provided in Scheme 1. As evident, the single pot synthesis gave the desired scaffold 4a, albeit in moderate yields, when compared to the step-wise process. The solvent choice seems to be crucial and better yields were observed by heating in NMP because of its polar aprotic nature and ease of removal during purification procedure. Best results obtained via either heating in single pot synthesis at 160 °C for 5 min (70% yield) or in step-wise synthesis by heating at 140 °C for 2 min for step 1 and then at 160 °C for 2 min for step 2 (89% yield).
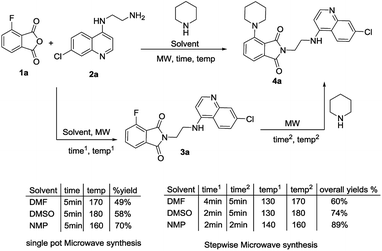 |
| | Scheme 1 Optimizing microwave-assisted synthesis of the C-4-piperidine ring substituted isoindoline-1,3-dione-4-aminoquinolines 4a. | |
Having optimized the reaction conditions, the scope of the developed strategy was then explored for the synthesis of a series of C-4/C-5 secondary amine linked isoindoline-1,3-dione-4-aminoquinolines 4b–4t following the stepwise microwave synthesis (Scheme 2). The structure assignment was done on the basis of spectral data and analytical evidences. For example, the compound 4s exhibited a molecular ion peak at m/z 508.2084 [M + H]+ in the High Resolution Mass Spectrum (HRMS). The noteworthy features of its 1H NMR spectrum encompassed the appearance of multiplet at δ 1.76–1.86, two triplets at 3.34 (J = 6.6 Hz) and 3.73 (J = 6.4 Hz) because of methylenes, two triplets at 2.66 (J = 4.7 Hz) and 3.40 (J = 4.6 Hz) because of piperazine ring protons and a doublet at δ 7.00 (J = 8.4 Hz) because of aromatic ring protons. The presence of absorption peaks at δ 169.1 and 168.7, corresponding to carbonyl carbons in the 13C NMR spectrum along with methylenes at δ 25.5, 26.5, 37.2 and 42.8 and piperazine carbons at δ 47.6 and 52.4, as corroborated by the 13C NMR (DEPT) spectrum, validated the assigned structure. For the synthesis of conjugates 7a–r, amide coupling of 6 with N-(7-chloroquinolin-4-yl)diamine 2 was done using amide coupling reagents EDC–HOBt at room temperature. Further, C-5 flouro substituted conjugates 7m–r were reacted with different secondary amines under microwave heating resulted in the formation of desired conjugates 8a–r in good to excellent yields (Scheme 3). Spectral data and analytical evidences were used to assign the structure to the synthesized conjugates.
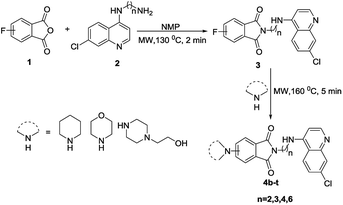 |
| | Scheme 2 Microwave-promoted synthesis of C-4/C-5 secondary amine substituted isoindoline-1,3-dione-4-aminoquinolines (4b–t). | |
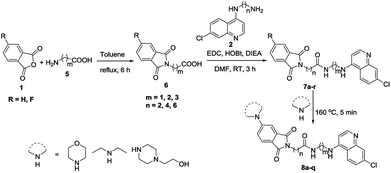 |
| | Scheme 3 Synthesis of C-5 substituted isoindoline-1,3-dione linked with 4-aminoquinolines via amide spacer 7a–r, 8a–r. | |
In vitro anti-mycobacterial evaluation
The synthesized scaffolds were assayed for their anti-mycobacterial activities against M. tuberculosis mc26230 and the result are enlisted in Table 1. As evident, all the synthesized compounds were not as active as isoniazid, but some of them showed promising activities. A closer analysis of Table 1 showed the dependence of activity on the nature of linker between two pharmacophores, the nature of substituent at C-4/C-5 position of isoindoline-1,3-dione as well as the linker used as spacer. Cytotoxic studies of all the synthesized compounds were also carried out against Vero cells so as to confirm whether the observed activities were due to their inherent anti-mycobacterial efficacy or cytotoxicity (Table 1, Fig. 2).
Table 1
In vitro anti-mycobacterial activity of synthesized compounds against M. tuberculosis mc26230 and cytotoxicity against Vero cells
| Code |
R |
m
|
n
|
MIC99a (μg mL−1) |
IC50 (μg mL−1) (cytotoxicity) |
SI |
|
The MIC expressed in μg mL−1 were determined using the microdilution method in broth medium; m, n are alkyl chain length (Schemes 1–3).
|
|
4a
|
4-Piperidyl |
— |
2 |
100 |
25.55 |
0.26 |
|
4b
|
4-Piperidyl |
— |
3 |
12.5 |
96.87 |
7.75 |
|
4c
|
4-Piperidyl |
— |
4 |
6.25 |
26.59 |
4.25 |
|
4d
|
4-Piperidyl |
— |
6 |
6.25 |
56.87 |
9.10 |
|
4e
|
5-Piperidyl |
— |
2 |
12.5 |
13.95 |
1.12 |
|
4f
|
5-Piperidyl |
— |
3 |
12.5 |
>100.4 |
>8 |
|
4g
|
5-Piperidyl |
— |
4 |
6.25 |
>100 |
>16 |
|
4h
|
5-Piperidyl |
— |
6 |
12.5 |
99.3 |
7.94 |
|
4i
|
4-Morpholinyl |
— |
2 |
100 |
28.43 |
0.28 |
|
4j
|
4-Morpholinyl |
— |
3 |
50 |
>100 |
>2 |
|
4k
|
4-Morpholinyl |
— |
4 |
12.5 |
36.05 |
2.88 |
|
4l
|
4-Morpholinyl |
— |
6 |
6.25 |
22.26 |
3.56 |
|
4m
|
5-Morpholinyl |
— |
2 |
50 |
12.15 |
0.24 |
|
4n
|
5-Morpholinyl |
— |
3 |
50 |
14.73 |
0.29 |
|
4o
|
5-Morpholinyl |
— |
4 |
12.5 |
11.30 |
0.904 |
|
4p
|
5-Morpholinyl |
— |
6 |
6.25 |
3.537 |
0.57 |
|
4q
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
— |
2 |
100 |
4.91 |
0.05 |
|
4r
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
— |
3 |
12.5 |
9.017 |
0.72 |
|
4s
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
— |
4 |
12.5 |
15.00 |
1.2 |
|
4t
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
— |
6 |
12.5 |
29.84 |
2.38 |
|
7a
|
H |
1 |
2 |
200 |
>100 |
>0.5 |
|
7b
|
H |
1 |
4 |
25 |
>100 |
>4 |
|
7c
|
H |
1 |
6 |
25 |
>100 |
>4 |
|
7d
|
H |
1 |
8 |
12.5 |
>100 |
>8 |
|
7e
|
H |
2 |
2 |
12.5 |
>100 |
>8 |
|
7f
|
H |
2 |
4 |
50 |
>100 |
>2 |
|
7g
|
H |
2 |
6 |
25 |
41.88 |
1.67 |
|
7h
|
H |
2 |
8 |
25 |
>100 |
>4 |
|
7i
|
H |
3 |
2 |
12.5 |
>100 |
>8 |
|
7j
|
H |
3 |
4 |
25 |
>100 |
>4 |
|
7k
|
H |
3 |
6 |
50 |
>100 |
>2 |
|
7l
|
H |
3 |
8 |
12.5 |
52.93 |
4.23 |
|
7m
|
5-F |
1 |
2 |
6.25 |
>100 |
>16 |
|
7n
|
5-F |
1 |
4 |
25 |
>100 |
>4 |
|
7o
|
5-F |
1 |
6 |
200 |
>100 |
>0.5 |
|
7p
|
5-F |
2 |
2 |
200 |
41.0 |
0.20 |
|
7q
|
5-F |
2 |
4 |
200 |
51.44 |
0.25 |
|
7r
|
5-F |
2 |
6 |
200 |
28.42 |
0.14 |
|
8a
|
5-Morpholinyl |
1 |
2 |
50 |
>100 |
>2 |
|
8b
|
5-Morpholinyl |
1 |
4 |
12.5 |
>100 |
>8 |
|
8c
|
5-Morpholinyl |
1 |
6 |
200 |
11.07 |
0.05 |
|
8d
|
5-Morpholinyl |
2 |
2 |
100 |
13.42 |
0.13 |
|
8e
|
5-Morpholinyl |
2 |
4 |
200 |
72.52 |
0.36 |
|
8f
|
5-Morpholinyl |
2 |
6 |
200 |
>100 |
>0.5 |
|
8g
|
5-(Diethylamino) |
1 |
2 |
50 |
21.28 |
0.42 |
|
8h
|
5-(Diethylamino) |
1 |
4 |
12.5 |
23.78 |
1.90 |
|
8i
|
5-(Diethylamino) |
1 |
6 |
100 |
>100 |
>1 |
|
8j
|
5-(Diethylamino) |
2 |
2 |
50 |
100 |
9.5 |
|
8k
|
5-(Diethylamino) |
2 |
4 |
200 |
44 |
0.22 |
|
8l
|
5-(Diethylamino) |
2 |
6 |
100 |
>100 |
>1 |
|
8m
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
1 |
2 |
50 |
>100 |
>2 |
|
8n
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
1 |
4 |
100 |
>100 |
>1 |
|
8o
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
1 |
6 |
200 |
>100 |
>0.5 |
|
8p
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
2 |
2 |
ND |
ND |
— |
|
8q
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
2 |
4 |
100 |
97.65 |
0.97 |
|
8r
|
5-(2-(Piperazin-1-yl)ethan-1-ol) |
2 |
6 |
25 |
>100 |
>4 |
| INH |
Isoniazid |
|
|
0.019 |
>100 |
|
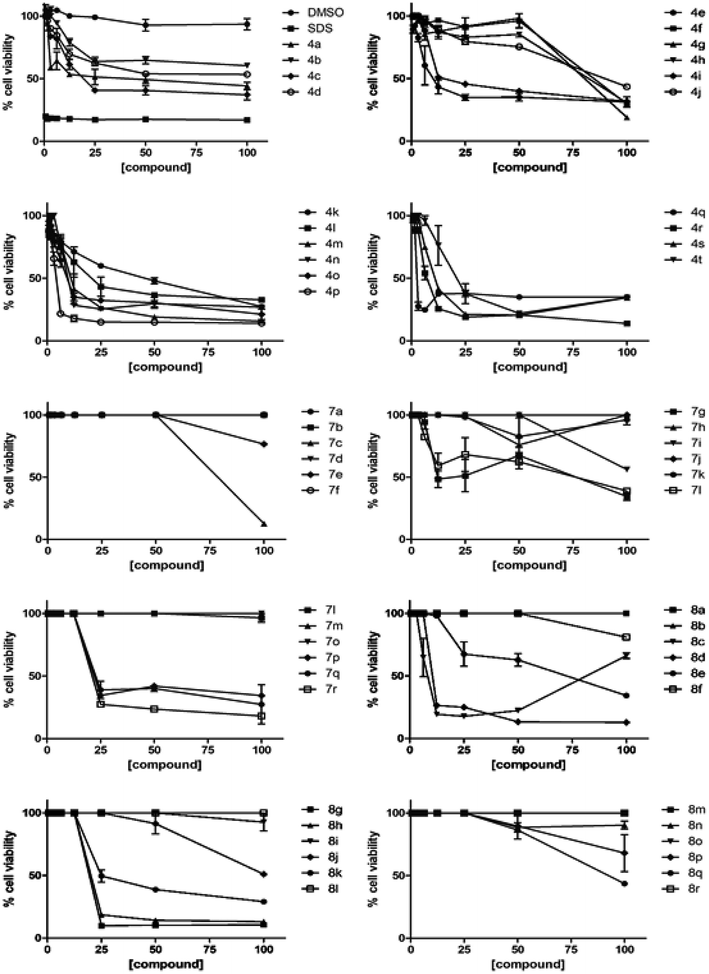 |
| | Fig. 2 Cytotoxicity of synthesized compounds (4a–t, 7a–r, 8a–r) on Vero cells. Shown are means and standard deviations calculated from two independent experiments. DMSO is included as a negative control, while SDS was included as a positive control. | |
Among isoindoline-1,3-dione-4-aminoquinolines linked via alkyl chain 4a–t, the replacement of fluoro-substituent (compound I, Fig. 1) with secondary amine on isoindoline-1,3-dione ring, not only improved the anti-TB activity but also reduced their cytotoxicity, which were in the range of 5.03–20.92 μg mL−1.26 Introduction of a piperidine ring at the C-4/C-5 position among compounds, 4a–4h revealed an increase in activity with the increase in alkyl chain length as evident by 4c (n = 4), 4d (n = 6) and 4g (n = 4), exhibiting MIC values of 6.25. Similar improvement in anti-TB activities was observed with the induction of morpholine ring. The compounds 4k, 4l, 4o and 4p exhibited MIC of 12.5, 6.25, 12.5, 6.25 μg mL−1 respectively. Introduction of hydroxy-ethyl-piperazine as the secondary amine counterpart considerably enhanced the anti-mycobacterial activity even at shorter chain lengths. 4r, 4s and 4t having propyl, butyl and hexyl spacer length along with hydroxyl-ethyl-piperazine at C-5 position exhibited MIC values of 12.5 μg mL−1, for all three compounds, but also increased their respective cytotoxicity.
Among the amide tethered isoindoline-1,3-dione-4-aminoquinoline 7a–r, although a decrease in anti-mycobacterial activity has been observed in general, the compounds were non-cytotoxic in nature. Among the scaffold having n = 1 (Glycine), the compound 7d (R = H, n = 8) and 7m (R = F, n = 2) displayed better activity profiles compared to the other members of the series with MIC of 12.5 and 6.25 μg mL−1 respectively. The introduction of β-alanine and γ-aminobutyric acid showed more or less similar activity profiles with low cytotoxicity. The introduction of secondary amine functionality among the amide-linked series resulted in considerable loss of anti-mycobacterial activities except for the compound 8b which was non-cytotoxic and exhibited a MIC of 12.5 μg mL−1. The generalized SAR of the synthesized isoindoline-1,3-dione-4-aminoquinolines (both the alkyl chain as well as amide-core tethered) for their anti-mycobacterial activity against mc26230 strain of M. tuberculosis and cytotoxicity against Vero cells is depicted in Fig. 3.
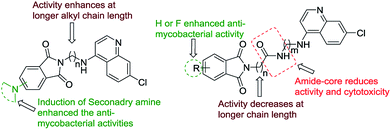 |
| | Fig. 3 Generalized SAR of synthesized isoindoline-1,3-dione-4-aminoquinolines | |
Conclusion
In conclusion, the present describes the microwave-promoted synthesis of a series of C-4/C-5 secondary amine substituted isoindoline-1,3-dione-4-aminoquinolines coupled via alkyl/amide core as linker. The synthesized scaffolds were evaluated for their anti-mycobacterial profiles against the M. tuberculosis mc26230 strain while cytotoxicity was determined against Vero cells. The compound 4g with an optimum combination of piperidine as the secondary amine at C-5 position and a butyl chain as spacer proved to be non-cytotoxic with a MIC99 of 6.25 μg mL−1.
Experimental section
General
Veego Precision Digital Melting Point apparatus (MP-D) was used for the determination of melting points which are uncorrected. 1H NMR spectra were recorded in deuterated chloroform (CDCl3)/DMSO-d6 using Bruker 500 (500 MHz) and Jeol 400 (400 MHz) spectrometers while TMS is used as internal standard. Microwave reactions were performed in a Biotage® Initiator+ instrument using sealed 2–5 mL process vials. Reaction times refer to irradiation time at the target temperature, not the total irradiation time. The temperature was measured with an IR sensor. Chemical shift values are specified as parts per million (ppm) downfield from TMS, while coupling constant (J) values are in hertz. Patterns of splitting are designated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double doublet, ddd: doublet of a doublet of a doublet, and br: broad peak. 13C NMR spectra were recorded on Bruker 125 MHz and Jeol 100 MHz spectrometers in CDCl3 and DMSO-d6. High Resolution Mass Spectra (HRMS) were recorded on a Bruker-micrOTOF-Q II spectrometer using ESI as the ion source.
General procedure for the preparation of C-4/C-5 secondary amine substituted isoindoline-1,3-dione-4-aminoquinolines (4a–t)
To a microwave reaction vial was added a solution of C-3/C-4 fluoro-phthalic anhydride (1.0 mmol) in 0.5 mL of NMP (N-methylpyrrolidin-2-one) and 4-aminoquinoline-diamines (1.0 mmol). After sealing with a PTFE cap, the vessel was heated to 130 °C for 2 min in the microwave reactor. After accomplishment of the first step, as obvious from TLC, secondary amine (1.2 mmol) was added in the same reaction vial. The reaction mixture was again heated at 160 °C for 5 min in the microwave reactor and the completion was ascertained using TLC. After completion, the contents were poured in water (20 mL) resulting in the precipitation of the desired product. The obtained product was filtered and re-crystallized using absolute ethanol.
2-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-4-(piperidin-1-yl)isoindoline-1,3-dione (4a).
Yield 89%; dark yellow solid; mp 140–141 °C; 1H NMR (500 MHz, CDCl3) δ 1.63–1.68 (m, 2H, –CH2–); 1.79–1.85 (m, 4H, 2× –CH2–); 3.36 (t, J = 5.2 Hz, 4H, 2× –CH2–); 3.35–3.47 (m, 2H, –CH2–); 3.78 (t, J = 6.4 Hz, 2H, –CH2–); 5.39 (s, 1H, NH-exchangeable with D2O); 6.41 (d, J = 5.2 Hz, 1H, Ar–H); 7.14 (d, J = 8.2 Hz, 1H, Ar–H); 7.32–7.37 (m, 2H, Ar–H); 7.54–7.58 (m, 1H, Ar–H); 7.82 (d, J = 9.0 Hz, 1H, Ar–H); 7.98 (s, 1H, Ar–H); 8.51 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.1, 25.7, 37.3, 43.2, 52.9, 99.1, 114.8, 117.3, 117.5, 121.3, 123.4, 125.3, 128.7, 134.6, 134.9, 135.2, 149.3, 149.9, 150.9, 152.1, 168.2, 168.8. HRMS calcd for C24H23ClN4O2 [M + H]+ 435.1543 found 435.1547.
2-(3-((7-Chloroquinolin-4-yl)amino)propyl)-4-(piperidin-1-yl)isoindoline-1,3-dione (4b).
Yield 88%; dark yellow solid; mp 121–122 °C; 1H NMR (500 MHz, CDCl3) δ 1.62–1.67 (m, 2H, –CH2–); 1.82–1.88 (m, 4H, 2× –CH2–); 1.98 (m, 2H, –CH2–), 3.32 (t, J = 5.1 Hz, 4H, 2× –CH2–); 3.35–3.40 (m, 2H, –CH2–); 3.80 (t, J = 6.4 Hz, 2H, –CH2–); 5.36 (s, 1H, NH-exchangeable with D2O); 6.40 (d, J = 5.1 Hz, 1H, Ar–H); 7.18 (d, J = 8.2 Hz, 1H, Ar–H); 7.32–7.36 (m, 2H, Ar–H); 7.51–7.55 (m, 1H, Ar–H); 7.81 (d, J = 8.8 Hz, 1H, Ar–H); 7.94 (s, 1H, Ar–H); 8.51 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.0, 25.5, 26.6, 37.2, 43.4, 52.7, 99.0, 114.6, 117.1, 117.4, 121.2, 123.4, 125.2, 128.8, 134.3, 134.8, 135.1, 149.4, 149.9, 150.8, 152.0, 168.1, 168.9. HRMS calcd for C25H25ClN4O2 [M + H]+ 449.1700 found 449.1708.
2-(4-((7-Chloroquinolin-4-yl)amino)butyl)-4-(piperidin-1-yl)isoindoline-1,3-dione (4c).
Yield 89%; dark brown solid; mp 110–111 °C; 1H NMR (500 MHz, CDCl3) δ 1.64–1.67 (m, 2H, –CH2–); 1.80–1.89 (m, 8H, 4× –CH2–); 3.30 (t, J = 5.3 Hz, 4H, 2× –CH2–); 3.37–3.41 (m, 2H, –CH2–); 3.77 (t, J = 6.5 Hz, 2H, –CH2–); 5.37 (s, 1H, NH-exchangeable with D2O); 6.41 (d, J = 5.4 Hz, 1H, Ar–H); 7.16 (d, J = 8.5 Hz, 1H, Ar–H); 7.33–7.37 (m, 2H, Ar–H); 7.53–7.56 (m, 1H, Ar–H); 7.80 (d, J = 8.9 Hz, 1H, Ar–H); 7.95 (s, 1H, Ar–H); 8.52 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.0, 25.6, 25.9, 26.5, 37.2, 43.0, 52.7, 99.0, 114.7, 117.2, 117.4, 121.2, 123.2, 125.2, 128.7, 134.4, 134.7, 135.0, 149.1, 149.7, 150.9, 152.0, 168.1, 168.7. HRMS calcd for C26H27ClN4O2 [M + H]+ 463.1856 found 463.1863.
2-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-4-(piperidin-1-yl)isoindoline-1,3-dione (4d).
Yield 90%; dark brown solid; mp 85–86 °C; 1H NMR (500 MHz, CDCl3) δ 1.40–1.56 (m, 4H, 2× –CH2–); 1.63–1.67 (m, 2H, –CH2–); 1.70–1.78 (m, 4H, 2× –CH2–); 1.80–1.83 (m, 4H, 2× –CH2–); 3.28–3.33 (m, 6H, 3× –CH2–); 3.68 (t, J = 5.5 Hz, 2H, –CH2–); 5.21 (s, 1H, NH-exchangeable with D2O); 6.41 (d, J = 5.4 Hz, 1H, Ar–H); 7.16 (d, J = 8.4 Hz, 1H, Ar–H); 7.33–7.37 (m, 2H, Ar–H); 7.52–7.55 (m, 1H, Ar–H); 7.76 (d, J = 8.9 Hz, 1H, Ar–H); 7.96 (s, 1H, Ar–H); 8.53 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.0, 25.9, 26.2, 26.3, 28.3, 28.5, 37.3, 42.8, 52.7, 99.0, 114.7, 117.2, 117.7, 121.0, 123.1, 125.2, 128.7, 134.5, 134.7, 134.9, 149.1, 149.7, 150.9, 152.0, 168.1, 168.7. HRMS calcd for C28H31ClN4O2 [M + H]+ 491.2169 found 491.2162.
2-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-5-(piperidin-1-yl)isoindoline-1,3-dione (4e).
Yield 85%; yellow solid; mp 180–181 °C; 1H NMR (500 MHz, CDCl3) δ 1.64–1.76 (m, 6H, 3× –CH2–); 3.37–3.44 (m, 6H, 3× –CH2–); 3.79 (t, J = 6.1 Hz, 2H, –CH2–); 6.11 (s, 1H, NH-exchangeable with D2O); 6.42 (d, J = 5.3 Hz, 1H, Ar–H); 7.03 (d, J = 8.0 Hz, 1H, Ar–H); 7.27 (s, 1H, Ar–H); 7.41 (d, J = 9.0 Hz, 1H, Ar–H); 7.67 (d, J = 8.6 Hz, 1H, Ar–H); 7.92–7.97 (m, 2H, Ar–H); 8.51 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.3, 25.3, 34.9, 39.7, 48.9, 98.8, 108.3, 117.2, 117.4, 118.4, 121.3, 125.1, 125.3, 128.7, 134.6, 134.8, 149.3, 149.6, 151.8, 155.4, 169.2, 169.7. HRMS calcd for C24H23ClN4O2 [M + H]+ 435.1543 found 435.1549.
2-(3-((7-Chloroquinolin-4-yl)amino)propyl)-5-(piperidin-1-yl)isoindoline-1,3-dione (4f).
Yield 88%; yellow solid; mp 97–98 °C; 1H NMR (500 MHz, CDCl3) δ 1.65–1.78 (m, 6H, 3× –CH2–); 2.00–2.05 (m, 2H, –CH2–); 3.39–3.45 (m, 6H, 3× –CH2–); 3.80 (t, J = 6.0 Hz, 2H, –CH2–); 6.12 (s, 1H, NH-exchangeable with D2O); 6.42 (d, J = 5.4 Hz, 1H, Ar–H); 7.01 (d, J = 8.0 Hz, 1H, Ar–H); 7.26 (s, 1H, Ar–H); 7.40 (d, J = 8.9 Hz, 1H, Ar–H); 7.65 (d, J = 8.5 Hz, 1H, Ar–H); 7.90–7.95 (m, 2H, Ar–H); 8.51 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.2, 25.2, 26.7, 34.6, 39.2, 48.8, 98.6, 108.2, 117.2, 117.4, 118.3, 121.4, 125.0, 125.3, 128.6, 134.5, 134.8, 149.2, 149.5, 151.9, 155.4, 169.1, 169.6. HRMS calcd for C25H25ClN4O2 [M + H]+ 449.1700 found 449.1712.
2-(4-((7-Chloroquinolin-4-yl)amino)butyl)-5-(piperidin-1-yl)isoindoline-1,3-dione (4g).
Yield 87%; yellow solid; mp 91–92 °C; 1H NMR (500 MHz, CDCl3) δ 1.41–1.49 (m, 4H, 2× –CH2–), 1.64–1.77 (m, 6H, 3× –CH2–); 3.37–3.44 (m, 6H, 3× –CH2–); 3.78 (t, J = 6.1 Hz, 2H, –CH2–); 6.11 (s, 1H, NH-exchangeable with D2O); 6.41 (d, J = 5.3 Hz, 1H, Ar–H); 7.00 (d, J = 8.1 Hz, 1H, Ar–H); 7.26 (s, 1H, Ar–H); 7.41 (d, J = 9.0 Hz, 1H, Ar–H); 7.64 (d, J = 8.6 Hz, 1H, Ar–H); 7.89–7.94 (m, 2H, Ar–H); 8.52 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.1, 25.2, 26.7, 26.9, 34.5, 39.2, 48.7, 98.4, 108.1, 117.2, 117.3, 118.2, 121.4, 125.1, 125.2, 128.7, 134.4, 134.7, 149.1, 149.5, 151.7, 155.3, 169.0, 169.5. HRMS calcd for C26H27ClN4O2 [M + H]+ 463.1856 found 463.1874.
2-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-5-(piperidin-1-yl)isoindoline-1,3-dione (4h).
Yield 89%; yellow solid; mp 88–89 °C; 1H NMR (500 MHz, CDCl3) δ 1.31–1.35 (m, 4H, 2× –CH2–), 1.52–1.56 (m, 2H, –CH2–), 1.63–1.78 (m, 8H, 4× –CH2–); 3.34–3.41 (m, 6H, 3× –CH2–); 3.75 (t, J = 6.2 Hz, 2H, –CH2–); 6.10 (s, 1H, NH-exchangeable with D2O); 6.40 (d, J = 5.3 Hz, 1H, Ar–H); 7.01 (d, J = 8.0 Hz, 1H, Ar–H); 7.24 (s, 1H, Ar–H); 7.40 (d, J = 9.0 Hz, 1H, Ar–H); 7.64 (d, J = 8.5 Hz, 1H, Ar–H); 7.88–7.94 (m, 2H, Ar–H); 8.51 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 24.2, 25.1, 26.7, 26.7, 28.3, 28.7, 34.3, 39.2, 48.4, 98.2, 108.0, 117.0, 117.2, 118.0, 121.2, 125.0, 125.2, 128.6, 134.5, 134.7, 149.1, 149.3, 151.6, 155.3, 169.1, 169.2. HRMS calcd for C28H31ClN4O2 [M + H]+ 491.2169 found 491.2178.
2-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-4-morpholinoisoindoline-1,3-dione (4i).
Yield 92%; yellow solid; mp 163–164 °C; 1H NMR (400 MHz, CDCl3) δ 3.36–3.40 (m, 6H, 3× –CH2–); 3.74 (t, J = 6.4 Hz, 2H, –CH2–); 3.95 (t, J = 4.5 Hz, 4H, 2× –CH2–); 5.30 (s, 1H, NH-exchangeable with D2O); 6.36 (d, J = 5.2 Hz, 1H, Ar–H); 7.11 (d, J = 8.3 Hz, 1H, Ar–H); 7.31–7.38 (m, 2H, Ar–H); 7.57–7.61 (m, 1H, Ar–H); 7.75 (d, J = 9.0 Hz, 1H, Ar–H); 7.88 (d, J = 2.0 Hz, 1H, Ar–H); 8.46 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (100 MHz) δ 37.5, 43.3, 51.7, 66.9, 99.0, 115.8, 117.1, 118.2, 121.4, 122.8, 125.4, 128.9, 134.4, 134.8, 135.6, 149.2, 149.8, 150.2, 152.1, 168.3, 168.6. HRMS calcd for C23H21ClN4O3 [M + H]+ 437.1336 found 437.1344.
2-(3-((7-Chloroquinolin-4-yl)amino)propyl)-4-morpholinoisoindoline-1,3-dione (4j).
Yield 90%; yellow solid; mp 141–142 °C; 1H NMR (400 MHz, CDCl3) δ 2.00–2.05 (m, 2H, –CH2–); 3.34–3.39 (m, 6H, 3× –CH2–); 3.71 (t, J = 6.5 Hz, 2H, –CH2–); 3.94 (t, J = 4.3 Hz, 4H, 2× –CH2–); 5.28 (s, 1H, NH-exchangeable with D2O); 6.35 (d, J = 5.3 Hz, 1H, Ar–H); 7.12 (d, J = 8.4 Hz, 1H, Ar–H); 7.30–7.37 (m, 2H, Ar–H); 7.56–7.59 (m, 1H, Ar–H); 7.75 (d, J = 8.9 Hz, 1H, Ar–H); 7.89 (d, J = 2.0 Hz, 1H, Ar–H); 8.45 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (100 MHz) δ 28.4, 37.3, 43.1, 51.6, 66.8, 99.0, 115.9, 117.1, 118.1, 121.3, 122.8, 125.4, 128.8, 134.5, 134.9, 135.6, 149.1, 149.8, 150.1, 152.1, 168.2, 168.7. HRMS calcd for C24H23ClN4O3 [M + H]+ 451.1492 found 451.1477.
2-(4-((7-Chloroquinolin-4-yl)amino)butyl)-4-morpholinoisoindoline-1,3-dione (4k).
Yield 89%; yellow solid; mp 110–111 °C; 1H NMR (400 MHz, CDCl3) δ 1.74–1.87 (m, 4H, 2× –CH2–); 3.30–3.37 (m, 6H, 3× –CH2–); 3.73 (t, J = 6.6 Hz, 2H, –CH2–); 3.91 (t, J = 4.5 Hz, 4H, 2× –CH2–); 5.29 (s, 1H, NH-exchangeable with D2O); 6.37 (d, J = 5.4 Hz, 1H, Ar–H); 7.12 (d, J = 8.4 Hz, 1H, Ar–H); 7.31–7.39 (m, 2H, Ar–H); 7.54–7.58 (m, 1H, Ar–H); 7.73 (d, J = 9.0 Hz, 1H, Ar–H); 7.91 (d, J = 2.1 Hz, 1H, Ar–H); 8.47 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz) δ 25.7, 26.5, 37.4, 43.0, 51.5, 66.9, 99.1, 115.9, 117.2, 118.1, 121.2, 122.8, 125.3, 128.7, 134.5, 134.9, 135.5, 149.0, 149.8, 150.1, 152.0, 168.1, 168.6. HRMS calcd for C25H25ClN4O3 [M + H]+ 465.1649 found 465.1665.
2-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-4-morpholinoisoindoline-1,3-dione (4l).
Yield 88%; yellow solid; mp 89–90 °C; 1H NMR (400 MHz, CDCl3) δ 1.35–1.39 (m, 4H, 2× –CH2–); 1.50–1.54 (m, 2H, –CH2–); 1.64–1.77 (m, 4H, 2× –CH2–); 3.29–3.36 (m, 6H, 3× –CH2–); 3.72 (t, J = 6.5 Hz, 2H, –CH2–); 3.90 (t, J = 4.6 Hz, 4H, 2× –CH2–); 5.28 (s, 1H, NH-exchangeable with D2O); 6.36 (d, J = 5.3 Hz, 1H, Ar–H); 7.11 (d, J = 8.5 Hz, 1H, Ar–H); 7.30–7.38 (m, 2H, Ar–H); 7.53–7.56 (m, 1H, Ar–H); 7.71 (d, J = 8.9 Hz, 1H, Ar–H); 7.90 (d, J = 2.0 Hz, 1H, Ar–H); 8.45 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz) δ 25.3, 26.6, 28.4, 28.8, 37.2, 43.2, 51.2, 66.7, 99.0, 115.8, 117.0, 118.0, 121.1, 122.6, 125.2, 128.7, 134.3, 134.7, 135.3, 149.1, 149.6, 150.2, 152.1, 168.0, 168.4. HRMS calcd for C27H29ClN4O3 [M + H]+ 493.1962 found 493.1971.
2-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-5-morpholinoisoindoline-1,3-dione (4m).
Yield 89%; dark brown solid; mp 192–193 °C; 1H NMR (500 MHz, CDCl3) δ 3.38 (t, J = 4.8 Hz, 4H, 2× –CH2–); 3.52–3.55 (m, 2H, –CH2–); 3.88 (t, J = 4.8 Hz, 4H, 2× –CH2–); 4.15 (t, J = 5.2 Hz, 2H, –CH2–); 6.13 (s, 1H, NH-exchangeable with D2O); 6.36 (d, J = 5.3 Hz, 1H, Ar–H); 7.05 (d, J = 8.4 Hz, 1H, Ar–H); 7.30 (s, 1H, Ar–H); 7.43 (d, J = 8.9 Hz, 1H, Ar–H); 7.73 (d, J = 8.4 Hz, 1H, Ar–H); 7.80 (d, J = 8.9 Hz, 1H, Ar–H); 7.94 (s, 1H, Ar–H); 8.52 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 36.7, 43.8, 47.5, 66.3, 98.6, 108.5, 117.1, 117.7, 120.1, 121.5, 125.2, 125.6, 128.5, 134.2, 134.9, 148.9, 149.7, 151.9, 155.6, 169.1, 169.5. HRMS calcd for C23H21ClN4O3 [M + H]+ 437.1336 found 437.1349.
2-(3-((7-Chloroquinolin-4-yl)amino)propyl)-5-morpholinoisoindoline-1,3-dione (4n).
Yield 85%; dark brown solid; mp 185–186 °C; 1H NMR (500 MHz, CDCl3) δ 1.65–1.70 (m, 2H, –CH2–); 3.36–3.45 (m, 6H, 3× –CH2–); 3.80 (t, J = 6.4 Hz, 2H, –CH2–); 3.90 (t, J = 4.7 Hz, 4H, 2× –CH2–); 5.30 (s, 1H, NH-exchangeable with D2O); 6.40 (d, J = 5.4 Hz, 1H, Ar–H); 7.04 (d, J = 8.4 Hz, 1H, Ar–H); 7.28 (s, 1H, Ar–H); 7.40 (d, J = 9.0 Hz, 1H, Ar–H); 7.70 (d, J = 8.3 Hz, 1H, Ar–H); 7.78 (d, J = 9.0 Hz, 1H, Ar–H); 7.94 (s, 1H, Ar–H); 8.52 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 26.8, 37.5, 43.0, 47.8, 66.2, 99.0, 108.4, 117.0, 117.6, 120.5, 121.4, 124.9, 125.4, 128.6, 134.3, 134.8, 149.0, 149.6, 152.0, 155.5, 168.9, 169.3. HRMS calcd for C24H23ClN4O3 [M + H]+ 451.1492 found 451.1486.
2-(4-((7-Chloroquinolin-4-yl)amino)butyl)-5-morpholinoisoindoline-1,3-dione (4o).
Yield 85%; dark brown solid; mp 180–181 °C; 1H NMR (500 MHz, CDCl3) δ 1.85–1.90 (m, 4H, 2× –CH2–); 3.36–3.41 (m, 6H, 3× –CH2–); 3.77 (t, J = 6.5 Hz, 2H, –CH2–); 3.89 (t, J = 4.8 Hz, 4H, 2× –CH2–); 5.29 (s, 1H, NH-exchangeable with D2O); 6.41 (d, J = 5.4 Hz, 1H, Ar–H); 7.04 (d, J = 8.4 Hz, 1H, Ar–H); 7.26 (s, 1H, Ar–H); 7.37 (d, J = 8.9 Hz, 1H, Ar–H); 7.68 (d, J = 8.4 Hz, 1H, Ar–H); 7.76 (d, J = 9.0 Hz, 1H, Ar–H); 7.95 (s, 1H, Ar–H); 8.52 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 25.6, 26.5, 37.3, 42.9, 47.7, 66.3, 99.1, 108.3, 117.2, 117.6, 120.7, 121.1, 124.8, 125.3, 128.7, 134.4, 134.8, 149.1, 149.7, 152.0, 155.4, 168.6, 169.0. HRMS calcd for C25H25ClN4O3 [M + H]+ 465.1649 found 465.1654.
2-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-5-morpholinoisoindoline-1,3-dione (4p).
Yield 89%; yellow solid; mp 177–178 °C; 1H NMR (500 MHz, CDCl3) δ 1.39–1.48 (m, 4H, 2× –CH2–); 1.60–1.69 (m, 4H, 2× –CH2–); 3.35–3.42 (m, 6H, 3× –CH2–); 3.76 (t, J = 6.2 Hz, 2H, –CH2–); 3.86 (t, J = 4.5 Hz, 4H, 2× –CH2–); 5.28 (s, 1H, NH-exchangeable with D2O); 6.40 (d, J = 5.3 Hz, 1H, Ar–H); 7.02 (d, J = 8.3 Hz, 1H, Ar–H); 7.25 (s, 1H, Ar–H); 7.35 (d, J = 8.8 Hz, 1H, Ar–H); 7.66 (d, J = 8.3 Hz, 1H, Ar–H); 7.76 (d, J = 8.8 Hz, 1H, Ar–H); 7.93 (s, 1H, Ar–H); 8.53 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (125 MHz, CDCl3) δ 25.5, 26.4, 28.7, 28.9, 37.2, 42.9, 47.6, 66.1, 99.0, 108.2, 117.2, 117.5, 120.8, 121.1, 124.7, 125.2, 128.6, 134.4, 134.7, 149.0, 149.6, 152.1, 155.3, 168.5, 169.1. HRMS calcd for C27H29ClN4O3 [M + H]+ 493.1962 found 493.1976.
2-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-5-(4-(2-hydroxyethyl)piperazin-1-yl)isoindoline-1,3-dione (4q).
Yield 88%; dark brown solid; mp 211–212 °C; 1H NMR (400 MHz, CDCl3) δ 2.65 (t, J = 5.4 Hz, 2H, –CH2); 2.69 (t, J = 5.0 Hz, 4H, 2× –CH2–); 3.39 (t, J = 6.4 Hz, 2H, –CH2–); 3.45 (t, J = 5.0 Hz, 4H, 2× –CH2–); 3.70 (t, J = 5.3 Hz, 2H, –CH2–); 3.77 (t, J = 6.5 Hz, 2H, –CH2–); 6.41 (d, J = 5.5 Hz, 1H, Ar–H); 7.04 (d, J = 8.3 Hz, 1H, Ar–H); 7.23 (s, 1H, Ar–H); 7.35 (d, J = 9.0 Hz, 1H, Ar–H); 7.65 (d, J = 8.2 Hz, 1H, Ar–H); 7.75 (d, J = 8.9 Hz, 1H, Ar–H); 7.93 (s, 1H, Ar–H); 8.49 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 37.4, 42.9, 47.8, 52.5, 57.9, 59.5, 99.1, 108.5, 117.0, 117.5, 120.1, 121.0, 124.9, 125.3, 128.9, 134.6, 134.8, 149.1, 149.8, 152.0, 155.3, 168.7, 169.1. HRMS calcd for C25H26ClN5O3 [M + H]+ 480.1758 found 480.1771.
2-(3-((7-Chloroquinolin-4-yl)amino)propyl)-5-(4-(2-hydroxyethyl)piperazin-1-yl)isoindoline-1,3-dione (4r).
Yield 89%; dark brown solid; mp 197–198 °C; 1H NMR (400 MHz, CDCl3) δ 1.99–2.05 (m, 2H, 2× –CH2–), 2.63 (t, J = 5.3 Hz, 2H, –CH2); 2.68 (t, J = 5.0 Hz, 4H, 2× –CH2–); 3.36 (t, J = 6.6 Hz, 2H, –CH2–); 3.43 (t, J = 5.1 Hz, 4H, 2× –CH2–); 3.68 (t, J = 5.4 Hz, 2H, –CH2–); 3.76 (t, J = 6.4 Hz, 2H, –CH2–); 6.39 (d, J = 5.3 Hz, 1H, Ar–H); 7.03 (d, J = 8.3 Hz, 1H, Ar–H); 7.22 (s, 1H, Ar–H); 7.33 (d, J = 9.0 Hz, 1H, Ar–H); 7.63 (d, J = 8.3 Hz, 1H, Ar–H); 7.74 (d, J = 8.9 Hz, 1H, Ar–H); 7.93 (s, 1H, Ar–H); 8.48 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 26.8, 37.8, 42.7, 47.7, 52.6, 57.6, 59.3, 99.1, 108.3, 117.2, 117.8, 120.2, 121.3, 124.9, 125.5, 128.6, 134.6, 134.9, 149.2, 149.8, 151.7, 155.4, 168.6, 169.2. HRMS calcd for C26H28ClN5O3 [M + H]+ 494.1914, found 494.2160.
2-(4-((7-Chloroquinolin-4-yl)amino)butyl)-5-(4-(2-hydroxyethyl)piperazin-1-yl)isoindoline-1,3-dione (4s).
Yield 87%; dark brown solid; mp 155–156 °C; 1H NMR (400 MHz, CDCl3) δ 1.76–1.86 (m, 4H, 2× –CH2–); 2.61 (t, J = 5.1 Hz, 2H, –CH2); 2.66 (t, J = 4.7 Hz, 4H, 2× –CH2–); 3.34 (t, J = 6.6 Hz, 2H, –CH2–); 3.40 (t, J = 4.6 Hz, 4H, 2× –CH2–); 3.66 (t, J = 5.1 Hz, 2H, –CH2–); 3.73 (t, J = 6.4 Hz, 2H, –CH2–); 6.37 (d, J = 5.3 Hz, 1H, Ar–H); 7.00 (d, J = 8.4 Hz, 1H, Ar–H); 7.22 (s, 1H, Ar–H); 7.34 (d, J = 8.9 Hz, 1H, Ar–H); 7.63 (d, J = 8.3 Hz, 1H, Ar–H); 7.73 (d, J = 8.9 Hz, 1H, Ar–H); 7.91 (s, 1H, Ar–H); 8.47 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 25.5, 26.5, 37.2, 42.8, 47.6, 52.4, 57.7, 59.3, 99.0, 108.4, 117.1, 117.6, 120.1, 121.1, 124.8, 125.3, 128.6, 134.4, 134.8, 149.0, 149.6, 151.9, 155.2, 168.7, 169.1. HRMS calcd for C27H30ClN5O3 [M + H]+ 508.2071 found 508.2084.
2-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-5-(4-(2-hydroxyethyl)piperazin-1-yl)isoindoline-1,3-dione (4t).
Yield 85%; dark brown solid; mp 93–94 °C; 1H NMR (400 MHz, CDCl3) δ 1.33–1.39 (m, 4H, 2× –CH2–); 1.75–1.88 (m, 4H, 2× –CH2–); 2.60 (t, J = 5.3 Hz, 2H, –CH2–); 2.65 (t, J = 4.8 Hz, 4H, 2× –CH2–); 3.33 (t, J = 6.5 Hz, 2H, –CH2–); 3.40 (t, J = 4.9 Hz, 4H, 2× –CH2–); 3.65 (t, J = 5.3 Hz, 2H, –CH2–); 3.72 (t, J = 6.5 Hz, 2H, –CH2–); 6.35 (d, J = 5.4 Hz, 1H, Ar–H); 7.01 (d, 8.4 Hz, 1H, Ar–H); 7.22 (s, 1H, Ar–H); 7.32 (d, J = 8.9 Hz, 1H, Ar–H); 7.61 (d, J = 8.4 Hz, 1H, Ar–H); 7.71 (d, J = 9.0 Hz, 1H, Ar–H); 7.90 (s, 1H, Ar–H); 8.45 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 24.4, 25.5, 26.5, 26.7, 28.3, 28.5, 37.0, 42.7, 47.4, 52.3, 57.5, 59.4, 99.0, 108.3, 117.0, 117.4, 120.1, 121.4, 124.6, 125.2, 128.7, 134.4, 134.6, 149.0, 149.8, 152.0, 155.1, 168.4, 169.0. HRMS calcd for C29H34ClN5O3 [M + H]+ 536.2384 found 536.2376.
General procedure for synthesis of amide linked substituted isoindoline-1,3-dione-4-aminoquinolines (7a–r, 8a–r)
Synthesis of C-5 substituted 2-(1,3-dioxoisoindolin-2-yl)acetic acid/3-(1,3-dioxoisoindolin-2-yl)propanoic acid/4-(1,3-dioxoisoindolin-2-yl)butanoic acid (6): C-4 substituted phthalic anhydride (1 mmol), amino acids (1.2 mmol) and triethylamine (Et3N) (1.2 mmol) were mixed in toluene and the reaction mixture was refluxed for 6 h. The progress of the reaction was monitored by thin layer chromatography (TLC). Toluene was evaporated under reduced pressure and the solid residue was stirred with 1 N-HCl in ice-cold water. The resulted white powder was filtered, dried and used for subsequent step without any purification.
Synthesis of C-5 substituted N-(2-((7-chloroquinolin-4-yl)amino)alkyl)-2-(1,3-dioxoisoindolin-2-yl)alkylamides (7a–r)
1.0 mmol of C-5 substituted (1,3-dioxoisoindolin-2-yl)acetic acid, N-ethyl-N-dimethylaminopropyl carbodiimide (EDC) (1.1 mmol), hydroxybenzotriazole (HOBt) (1.2 mmol) and N,N-diisopropylethylamine (2.0 mmol) were mixed in minimum DMF and the obtained mixture was stirred for 5 min. Then, 4-aminoquinoline-diamines (1.0 mmol) was added to the reaction mixture and the stirring was continued for 5 h. The reaction end was proved by thin layer chromatography (TLC). Then, DMF was evaporated using rotary evaporator and cold water (20 mL) was added, and solid precipitates obtained were filtered and washed with cold water. The crude product was recrystallized in absolute ethanol.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-2-(1,3-dioxoisoindolin-2-yl)acetamide (7a).
Yield 82%; white solid; mp 189–190 °C; 1H NMR (400 MHz, DMSO-d6) δ 3.31 (s, 4H, 2× –CH2–); 4.18 (s, 2H, –CH2–); 6.50 (d, J = 5.4 Hz, 1H, Ar–H); 7.33 (t, J = 5.0 Hz, 1H, NH-exchangeable with D2O); 7.38 (dd, J = 1.8, 9.0 Hz, 1H, Ar–H); 7.74 (d, J = 2.0 Hz, 1H, Ar–H); 7.82–7.88 (m, 4H, Ar–H); 8.11 (d, J = 9.0 Hz, 1H, Ar–H); 8.36 (d, J = 5.0 Hz, 1H, Ar–H); 8.45 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 13C NMR (100 MHz, DMSO-d6) δ 37.9, 40.7, 42.3, 99.1, 117.9, 123.7, 124.4, 124.6, 128.0, 132.2, 133.9, 135.1, 149.5, 150.5, 152.4, 167.2, 168.0. HRMS calcd for C21H17ClN4O3 [M + H]+ 409.1023 found 409.1041.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-2-(1,3-dioxoisoindolin-2-yl)acetamide (7b).
Yield 81%; white solid; mp 129–130 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.96–1.29 (m, 4H, 2× –CH2–); 3.08–3.15 (m, 2H, –CH2–); 3.25–3.29 (m, 2H, –CH2–); 4.16 (s, 2H, –CH2–); 6.46 (d, J = 5.5 Hz, 1H, Ar–H); 7.33 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 7.42 (dd, J = 2.4, 9.0 Hz, 1H, Ar–H); 7.76 (d, J = 2.3 Hz, 1H, Ar–H); 7.83–7.90 (m, 4H, Ar–H); 8.20–8.30 (m, 2H, Ar–H + NH-exchangeable with D2O); 8.37 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 25.4, 27.2, 37.2, 40.2, 42.1, 99.2, 117.8, 123.7, 124.3, 124.7, 128.1, 132.2, 133.8, 135.2, 149.4, 150.4, 152.5, 167.0, 168.1. HRMS calcd for C23H21ClN4O3 [M + H]+ 437.1336 found 437.1323.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-2-(1,3-dioxoisoindolin-2-yl)acetamide (7c).
Yield 85%; white solid; mp 110–111 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.32–1.41 (m, 6H, 3× –CH2–); 1.57–1.64 (m, 2H, –CH2–); 3.00–3.05 (m, 2H, –CH2–); 3.20–3.25 (m, 2H, –CH2–); 4.13 (s, 2H, –CH2–); 6.44 (d, J = 5.6 Hz, 1H, Ar–H); 7.38–7.42 (m, 2H, Ar–H + NH-exchangeable with D2O); 7.74 (d, J = 2.2 Hz, 1H, Ar–H); 7.80–7.86 (m, 4H, Ar–H); 8.16 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 8.25 (d, J = 9.0 Hz, 1H, Ar–H); 8.34 (d, J = 5.5 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 26.5, 26.7, 28.2, 29.4, 39.1, 40.6, 42.9, 99.1, 117.8, 123.6, 124.7, 127.8, 132.3, 134.2, 135.0, 148.8, 150.9, 151.7, 166.3, 168.1. HRMS calcd for C25H25ClN4O3 [M + H]+ 465.1649 found 465.1629.
N-(8-((7-Chloroquinolin-4-yl)amino)octyl)-2-(1,3-dioxoisoindolin-2-yl)acetamide (7d).
Yield 76%; white solid; mp 101–102 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.22–1.41 (m, 12H, 6× –CH2–); 3.03–3.07 (m, 2H, –CH2–); 3.22–3.26 (m, 2H, –CH2–); 4.17 (s, 2H, –CH2–); 6.45 (d, J = 5.3 Hz, 1H, Ar–H); 7.29 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 7.43 (dd, J = 2.3, 8.9 Hz, 1H, Ar–H); 7.77 (d, J = 2.3 Hz, 1H, Ar–H); 7.84–7.90 (m, 4H, Ar–H); 8.18 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 8.27 (d, J = 9.0 Hz, 1H, Ar–H); 8.38 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 26.4, 26.6, 28.2, 28.4, 29.3, 29.6, 37.8, 40.6, 42.8, 99.2, 117.9, 123.4, 124.3, 127.9, 132.1, 134.2, 135.2, 148.7, 150.6, 151.4, 166.1, 168.0. HRMS calcd for C27H29ClN4O3 [M + H]+ 493.1962 found 493.1956.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-3-(1,3-dioxoisoindolin-2-yl)propanamide (7e).
Yield 81%; white solid; mp 182–183 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.42 (t, J = 7.2 Hz, 2H, –CH2–); 2.25–2.30 (m, 4H, 2× –CH2–); 3.75 (t, J = 7.4 Hz, 2H, –CH2–); 6.56 (d, J = 5.8 Hz, 1H, Ar–H); 7.45 (dd, J = 2.0, 9.0 Hz, 1H, Ar–H); 7.70–7.74 (m, 4H, Ar–H); 7.79 (d, J = 2.3 Hz, 1H, Ar–H); 7.93 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 8.17 (d, J = 9.1 Hz, 1H, Ar–H); 8.29 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 8.40 (t, J = 5.8 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 34.6, 34.9, 37.6, 42.9, 99.0, 117.2, 123.4, 124.8, 125.4, 126.7, 132.0, 134.7, 135.3, 146.2, 149.6, 152.2, 168.1, 170.9. HRMS calcd for C22H19ClN4O3 [M + H]+ 423.1179 found 423.1161.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-3-(1,3-dioxoisoindolin-2-yl)propanamide (7f).
Yield 77%; white solid; mp 172–173 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.23–1.33 (m, 4H, 2× –CH2–); 2.41 (t, J = 7.1 Hz, 2H, –CH2–); 3.05–3.14 (m, 2H, –CH2–); 3.22–3.27 (m, 2H, –CH2–); 3.73 (t, J = 7.2 Hz, 2H, –CH2–); 6.45 (d, J = 5.4 Hz, 1H, Ar–H); 7.32 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 7.43 (dd, J = 2.2, 8.9 Hz, 1H, Ar–H); 7.74 (d, J = 2.2 Hz, 1H, Ar–H); 7.83–7.91 (m, 4H, Ar–H); 8.19–8.28 (m, 2H, Ar–H + NH-exchangeable with D2O); 8.38 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 25.4, 27.1, 34.6, 38.2, 40.8, 42.2, 99.2, 117.5, 123.6, 124.3, 124.8, 128.3, 132.5, 133.7, 135.2, 149.6, 150.2, 152.6, 168.1, 170.8. HRMS calcd for C24H23ClN4O3 [M + H]+ 451.1492 found 451.1466.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-3-(1,3-dioxoisoindolin-2-yl)propanamide (7g).
Yield 79%; white solid; mp 122–123 °C; 1H NMR (400 MHz, DMSO-d6) 1.29–1.40 (m, 6H, 3× –CH2–); 1.55–1.63 (m, 2H, –CH2–); 2.40 (t, J = 7.2 Hz, 2H, –CH2–); 3.01–3.07 (m, 2H, –CH2–); 3.21–3.27 (m, 2H, –CH2–); 3.72 (t, J = 7.2 Hz, 2H, –CH2–); 6.44 (d, J = 5.3 Hz, 1H, Ar–H); 7.30 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 7.42 (dd, J = 2.2, 9.0 Hz, 1H, Ar–H); 7.73 (d, J = 2.1 Hz, 1H, Ar–H); 7.83–7.92 (m, 4H, Ar–H); 8.20–8.29 (m, 2H, Ar–H + NH-exchangeable with D2O); 8.35 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 26.4, 26.7, 28.3, 29.1, 34.6, 39.4, 40.8, 42.2, 99.1, 117.7, 123.6, 124.2, 124.8, 128.4, 132.4, 133.7, 135.1, 149.5, 150.1, 152.3, 168.1, 170.7. HRMS calcd for C26H27ClN4O3 [M + H]+ 479.1805 found 479.1814.
N-(8-((7-Chloroquinolin-4-yl)amino)octyl)-3-(1,3-dioxoisoindolin-2-yl)propanamide (7h).
Yield 70%; white solid; mp 85–86 °C; 1H NMR (400 MHz, DMSO-d6) 1.20–1.40 (m, 12H, 6× –CH2–); 2.41 (t, J = 7.1 Hz, 2H, –CH2–); 3.02–3.07 (m, 2H, –CH2–); 3.21–3.26 (m, 2H, –CH2–); 3.71 (t, J = 7.2 Hz, 2H, –CH2–); 6.43 (d, J = 5.3 Hz, 1H, Ar–H); 7.31 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 7.41 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.75 (d, J = 2.0 Hz, 1H, Ar–H); 7.85–7.94 (m, 4H, Ar–H); 8.20–8.30 (m, 2H, Ar–H + NH-exchangeable with D2O); 8.34 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 26.2, 26.6, 28.2, 28.5, 29.1, 29.5, 34.6, 37.8, 40.5, 42.2, 99.2, 117.5, 123.8, 124.2, 124.9, 128.5, 132.4, 133.9, 135.0, 149.5, 150.0, 152.5, 168.3, 170.9. HRMS calcd for C28H31ClN4O3 [M + H]+ 507.2118 found 507.2129.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-4-(1,3-dioxoisoindolin-2-yl)butanamide (7i).
Yield 83%; white solid; mp 177–178 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.77–1.83 (m, 2H, –CH2–); 2.12 (t, J = 7.5 Hz, 2H, –CH2–); 3.32 (t, J = 6.0 Hz, 2H, –CH2–); 3.52–3.57 (m, 4H, 2× –CH2–); 6.89 (d, J = 7.1 Hz, 1H, Ar–H); 7.77 (dd, J = 2.2, 9.1 Hz, 1H, Ar–H); 7.81–7.86 (m, 4H, Ar–H); 8.00 (d, J = 2.1 Hz, 1H, Ar–H); 8.16 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 8.53–8.57 (m, 2H, Ar–H); 9.54 (t, J = 5.9 Hz, 1H, NH-exchangeable with D2O); 13C NMR (125 MHz, DMSO-d6) δ 24.3, 33.1, 37.5, 37.6, 43.2, 98.9, 115.9, 119.4, 123.4, 126.1, 127.3, 132.0, 134.8, 138.4, 138.9, 143.3, 156.1, 168.3, 172.6. HRMS calcd for C23H21ClN4O3 [M + H]+ 437.1336 found 437.1321.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-4-(1,3-dioxoisoindolin-2-yl)butanamide (7j).
Yield 74%; white solid; mp 145–146 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.22–1.34 (m, 4H, 2× –CH2–); 1.76–1.83 (m, 2H, –CH2–); 2.11 (t, J = 7.1 Hz, 2H, –CH2–); 3.08–3.15 (m, 2H, –CH2–); 3.25–3.29 (m, 2H, –CH2–); 3.56 (t, J = 7.2 Hz, 2H, –CH2–); 6.85 (d, J = 7.1 Hz, 1H, Ar–H); 7.76 (dd, J = 2.0, 9.0 Hz, 1H, Ar–H); 7.80–7.86 (m, 4H, Ar–H); 8.01 (d, J = 2.1 Hz, 1H, Ar–H); 8.14 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 8.54–8.59 (m, 2H, Ar–H); 8.85 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 13C NMR (125 MHz, DMSO-d6) δ 24.4, 25.3, 27.5, 33.4, 37.6, 40.9, 43.2, 99.1, 115.7, 119.3, 123.4, 126.4, 127.3, 132.1, 134.8, 138.5, 138.8, 143.4, 156.1, 168.2, 172.4. HRMS calcd for C25H25ClN4O3 [M + H]+ 465.1649 found 465.1633.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-4-(1,3-dioxoisoindolin-2-yl)butanamide (7k).
Yield 75%; white solid; mp 158–159 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.18–1.25 (m, 2H, –CH2–); 1.27–1.40 (m, 4H, 2× –CH2–); 1.61–1.67 (m, 2H, –CH2–); 1.78–1.85 (m, 2H, –CH2–); 2.06–2.10 (m, 2H, –CH2–); 2.91–2.99 (m, 2H, –CH2–); 3.23–3.27 (m, 2H, –CH2–); 3.57 (t, J = 7.1 Hz, 2H, –CH2–); 6.46 (d, J = 5.4 Hz, 1H, Ar–H); 7.32 (t, J = 5.55 Hz, 1H, NH-exchangeable with D2O); 7.44 (dd, J = 2.1, 8.9 Hz, 1H, Ar–H); 7.71 (d, J = 5.5 Hz, 1H, Ar–H); 7.76 (d, J = 2.3 Hz, 1H, Ar–H); 7.81–7.86 (m, 4H, Ar–H); 8.28 (d, J = 9.0 Hz, 1H, Ar–H); 8.38 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 13C NMR (125 MHz, DMSO-d6) δ 24.5, 26.4, 26.9, 28.2, 29.7, 33.5, 38.4, 40.4, 43.5, 99.2, 115.6, 119.3, 123.5, 126.4, 127.2, 132.0, 134.9, 138.5, 138.9, 143.3, 156.2, 168.1, 172.2. HRMS calcd for C27H29ClN4O3 [M + H]+ 493.1962 found 4931951.
N-(8-((7-Chloroquinolin-4-yl)amino)octyl)-4-(1,3-dioxoisoindolin-2-yl)butanamide (7l).
Yield 78%; white solid; mp 152–153 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.20–1.39 (m, 12H, 6× –CH2–); 1.75–1.84 (m, 2H, –CH2–); 2.07–2.11 (m, 2H, –CH2–); 3.02–3.06 (m, 2H, –CH2–); 3.20–3.25 (m, 2H, –CH2–); 3.54 (t, J = 7.2 Hz, 2H, –CH2–); 6.44 (d, J = 5.2 Hz, 1H, Ar–H); 7.28 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 7.44 (dd, J = 2.2, 8.9 Hz, 1H, Ar–H); 7.75 (d, J = 2.3 Hz, 1H, Ar–H); 7.83–7.90 (m, 4H, Ar–H); 8.19 (d, J = 5.4 Hz, 1H, Ar–H); 8.27 (d, J = 8.9 Hz, 1H, Ar–H); 8.40 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 13C NMR (125 MHz, DMSO-d6) δ 24.5, 26.4, 26.7, 28.1, 28.5, 29.4, 29.8, 33.3, 37.8, 40.5, 43.4, 99.1, 115.9, 119.34, 123.4, 126.6, 127.2, 132.1, 134.6, 138.7, 138.8, 143.3, 156.3, 168.2, 172.6. HRMS calcd for C29H33ClN4O3 [M + H]+ 521.2275 found 521.2258.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-2-(5-fluoro-1,3-dioxoisoindolin-2-yl)acetamide (7m).
Yield 81%; white solid; mp 192–193 °C; 1H NMR (DMSO-d6, 500 MHz): 3.31 (s, 4H, 2× –CH2–); 4.17 (s, 2H, –CH2–); 6.51 (d, J = 5.5 Hz, 1H, Ar–H); 7.37–7.42 (m, 2H, Ar–H + NH-exchangeable with D2O); 7.64–7.69 (m, 1H, Ar–H); 7.75 (d, J = 2.2 Hz, 1H, Ar–H); 7.77–7.80 (m, 1H, Ar–H); 7.93–7.96 (m, 1H, Ar–H); 8.13 (d, J = 9.0 Hz, Ar–H); 8.37 (d, J = 5.5 Hz, Ar–H); 8.45 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 13C NMR (125 MHz, DMSO-d6) δ 36.7, 41.3, 42.5, 99.1, 111.3 (d, J = 22.9 Hz), 117.8, 121.5 (d, J = 23.7 Hz), 124.8, 125.1, 126.7 (d, J = 9.6 Hz), 127.9, 128.5 (d, J = 1.9 Hz), 133.4, 135.1 (d, J = 9.4 Hz), 149.7, 150.6, 152.5, 166.2 (d, J = 252.3 Hz), 166.7 (d, J = 2.3 Hz), 167.3, 169.7. HRMS calcd for C21H16ClFN4O3 [M + H]+ 427.0929 found 427.0935.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-2-(5-fluoro-1,3-dioxoisoindolin-2-yl)acetamide (7n).
Yield 85%; white solid; mp 140–141 °C; 1H NMR (DMSO-d6, 500 MHz): 1.50–1.56 (m, 2H, –CH2–); 1.62–1.68 (m, 2H, –CH2–); 3.11–3.15 (m, 2H, –CH2–); 3.27–3.31 (m, 2H, –CH2–); 4.18 (s, 2H, –CH2–); 6.50 (d, J = 5.6 Hz, 1H, Ar–H); 7.45–7.47 (m, 2H, Ar–H + NH-exchangeable with D2O); 7.68–7.72 (m, 1H, Ar–H); 7.78 (d, J = 2.3 Hz, 1H, Ar–H); 7.81–7.83 (m, 1H, Ar–H); 7.97–7.99 (m, 1H, Ar–H); 8.25 (t, J = 5.7 Hz, NH-exchangeable with D2O); 8.29 (d, J = 9.0 Hz, Ar–H); 8.39 (d, J = 5.5 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 25.7, 26.8, 35.2, 39.3, 42.9, 99.0, 111.7 (d, J = 23.3 Hz), 117.7, 121.5 (d, J = 23.4 Hz), 124.4, 124.8, 126.6 (d, J = 9.6 Hz), 127.9, 128.3 (d, J = 1.8 Hz), 133.9, 135.2 (d, J = 9.4 Hz), 149.1, 150.4, 152.2, 166.1 (d, J = 251.6 Hz), 166.9 (d, J = 2.3 Hz), 167.4, 169.8. HRMS calcd for C23H20ClFN4O3 [M + H]+ 455.1242 found 455.1265.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-2-(5-fluoro-1,3-dioxoisoindolin-2-yl)acetamide (7o).
Yield 84%; white solid; mp 131–132 °C; 1H NMR (DMSO-d6, 400 MHz): 1.36–1.44 (m, 4H, 2× –CH2–); 1.54–1.62 (m, 4H, 2× –CH2–); 3.01–3.07 (m, 2H, –CH2–); 3.22–3.25 (m, 2H, –CH2–); 4.17 (s, 2H, –CH2–); 6.39 (d, J = 5.3 Hz, 1H, Ar–H); 7.30 (t, J = 5.4 Hz, NH-exchangeable with D2O); 7.35 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.54–7.59 (m, 1H, Ar–H); 7.69–7.74 (m, 2H, Ar–H); 7.88–7.90 (m, 1H, Ar–H); 7.96 (t, J = 5.5 Hz, NH-exchangeable with D2O); 8.22 (d, J = 9.0 Hz, Ar–H); 8.31 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 26.5, 26.9, 28.5, 29.6, 39.5, 40.9, 42.5, 99.1, 111.4 (d, J = 23.7 Hz), 117.7, 121.9 (d, J = 23.7 Hz), 124.9, 125.4, 126.5 (d, J = 9.7 Hz), 127.8, 128.2 (d, J = 1.9 Hz), 133.7, 135.1 (d, J = 9.7 Hz), 149.6, 150.7, 152.3, 166.0 (d, J = 252.3 Hz), 166.8 (d, J = 2.4 Hz), 167.2, 169.6. HRMS calcd for C25H24ClFN4O3 [M + H]+ 483.1555 found 483.1561.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-3-(5-fluoro-1,3-dioxoisoindolin-2-yl)propanamide (7p).
Yield 81%; white solid; mp 183–184 °C; 1H NMR (DMSO-d6, 400 MHz): 2.40 (t, J = 7.2 Hz, 2H, –CH2–); 3.00–3.09 (m, 4H, 2× –CH2–); 3.73 (t, J = 7.1 Hz, 2H, –CH2–); 6.41 (d, J = 5.3 Hz, 1H, Ar–H); 7.29 (t, J = 5.5 Hz, NH-exchangeable with D2O); 7.41 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.56–7.62 (m, 1H, Ar–H); 7.70–7.74 (m, 2H, Ar–H); 7.84–7.88 (m, 1H, Ar–H); 7.99 (t, J = 5.4 Hz, NH-exchangeable with D2O); 8.22 (d, J = 9.1 Hz, Ar–H); 8.35 (d, J = 5.5 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 34.5, 37.9, 40.8, 42.4, 99.2, 111.3 (d, J = 23.2 Hz), 117.5, 121.2 (d, J = 23.4 Hz), 124.4, 124.8, 126.5 (d, J = 9.5 Hz), 127.8, 128.2 (d, J = 1.8 Hz), 133.7, 135.0 (d, J = 9.5 Hz), 149.6, 150.3, 152.2, 166.1 (d, J = 251.9 Hz), 166.5 (d, J = 2.3 Hz), 167.1, 169.9. HRMS calcd for C27H29ClN4O3 [M]+ 492.1928 found 4921941. HRMS calcd for C22H18ClFN4O3 [M + H]+ 441.1085 found 441.1097.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-3-(5-fluoro-1,3-dioxoisoindolin-2-yl)propanamide (7q).
Yield 76%; white solid; mp 177–178 °C; 1H NMR (DMSO-d6, 400 MHz): 1.38–1.45 (m, 2H, –CH2–); 1.51–1.58 (m, 2H, –CH2–); 2.38 (t, J = 7.2 Hz, 2H, –CH2–); 2.98–3.03 (m, 2H, –CH2–); 3.15–3.20 (m, 2H, –CH2–); 3.72 (t, J = 7.3 Hz, 2H, –CH2–); 6.39 (d, J = 5.4 Hz, 1H, Ar–H); 7.28 (t, J = 5.4 Hz, NH-exchangeable with D2O); 7.39 (dd, J = 2.3, 9.1 Hz, 1H, Ar–H); 7.54–7.59 (m, 1H, Ar–H); 7.69–7.72 (m, 2H, Ar–H); 7.85–7.88 (m, 1H, Ar–H); 7.98 (t, J = 5.5 Hz, NH-exchangeable with D2O); 8.21 (d, J = 9.0 Hz, Ar–H); 8.33 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 25.6, 27.1, 34.4, 35.1, 38.6, 42.5, 99.1, 111.4 (d, J = 23.5 Hz), 117.9, 121.5 (d, J = 23.5 Hz), 124.5, 124.6, 126.2 (d, J = 9.6 Hz), 127.8, 128.3 (d, J = 1.7 Hz), 133.9, 135.1 (d, J = 9.5 Hz), 149.4, 150.6, 152.3, 166.2 (d, J = 251.7 Hz), 166.8 (d, J = 2.4 Hz), 167.2, 169.8. HRMS calcd for C24H22ClFN4O3 [M + H]+ 469.1398 found 469.1387.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-3-(5-fluoro-1,3-dioxoisoindolin-2-yl)propanamide (7r).
Yield 77%; white solid; mp 138–139 °C; 1H NMR (DMSO-d6, 400 MHz): 1.32–1.39 (m, 6H, 3× –CH2–); 1.54–1.63 (m, 2H, –CH2–); 2.39 (t, J = 7.1 Hz, 2H, –CH2–); 3.00–3.05 (m, 2H, –CH2–); 3.19–3.24 (m, 2H, –CH2–); 3.71 (t, J = 7.2 Hz, 2H, –CH2–); 6.38 (d, J = 5.3 Hz, 1H, Ar–H); 7.29 (t, J = 5.4 Hz, NH-exchangeable with D2O); 7.37 (dd, J = 2.2, 9.0 Hz, 1H, Ar–H); 7.53–7.59 (m, 1H, Ar–H); 7.68–7.73 (m, 2H, Ar–H); 7.86–7.89 (m, 1H, Ar–H); 7.95 (t, J = 5.4 Hz, NH-exchangeable with D2O); 8.20 (d, J = 9.1 Hz, Ar–H); 8.32 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 26.7, 26.9, 28.3, 29.5, 34.1, 39.3, 40.7, 42.4, 99.0, 111.5 (d, J = 23.4 Hz), 117.5, 121.8 (d, J = 23.7 Hz), 124.8, 125.3, 126.1 (d, J = 9.7 Hz), 127.9, 128.2 (d, J = 1.9 Hz), 133.8, 135.0 (d, J = 9.7 Hz), 149.5, 150.5, 152.2, 166.1 (d, J = 251.6 Hz), 166.9 (d, J = 2.5 Hz), 167.3, 169.7. HRMS calcd for C26H26ClFN4O3 [M + H]+ 497.1711 found 497.1723.
General procedure for synthesis of C-5 secondary amine substituted N-((7-chloroquinolin-4-yl)aminoalkyl)-2-(1,3-dioxoisoindolin-2-yl)alkylamides 8a–r
In a microwave reaction vial was added a solution of C-5 fluoro substituted N-((7-chloroquinolin-4-yl)aminoalkyl)-2-(1,3-dioxoisoindolin-2-yl)alkylamides (1.0 mmol) in 0.5 mL of NMP (N-methylpyrrolidin-2-one) and secondary amines (1.2 mmol). After sealing with a PTFE cap, the vessel was heated to 160 °C for 5 min in the microwave reactor and the completion was ascertained using TLC. After completion, the contents were poured in water (20 mL) resulting in the precipitation of the desired product. The yellow product obtained was filtered and re-crystallized using absolute ethanol.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-2-(5-morpholino-1,3-dioxoisoindolin-2-yl)acetamide (8a).
Yield 76%; yellow solid; mp 216–217 °C; 1H NMR (500 MHz, DMSO-d6) δ 3.30–3.32 (m, 6H, 3× –CH2–); 3.59–3.63 (m, 2H, –CH2–); 3.74–3.76 (m, 4H, 2× –CH2–); 4.12 (s, 2H, –CH2–); 6.90 (d, J = 7.1 Hz, 1H, Ar–H); 7.25 (dd, J = 2.3, 8.5 Hz, 1H, Ar–H); 7.32 (d, J = 2.2 Hz, 1H, Ar–H); 7.66 (d, J = 8.4 Hz, 1H, Ar–H); 7.77 (dd, J = 2.0, 9.0 Hz, 1H, Ar–H); 8.05 (d, J = 2.1 Hz, 1H, Ar–H); 8.55–8.57 (m, 3H, Ar–H); 9.55 (t, J = 5.9 Hz, 1H, NH-exchangeable with D2O); 13C NMR (125 MHz, DMSO-d6) δ 39.1, 42.7, 47.6, 48.8, 66.5, 99.1, 108.3, 117.8, 118.1, 120.2, 124.5, 124.6, 125.1, 127.9, 133.5, 134.6, 149.4, 150.5, 152.3, 155.7, 166.4, 167.7, 168.4, 174.1. HRMS calcd for C25H24ClN5O4 [M + H]+ 494.1550 found 494.1564.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-2-(5-morpholino-1,3-dioxoisoindolin-2-yl)acetamide (8b).
Yield 79%; yellow solid; mp 174–175 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.51–1.56 (m, 2H, –CH2–); 1.63–1.69 (m, 2H, –CH2–); 3.07–3.011 (m, 2H, –CH2–); 3.25–3.28 (m, 2H, –CH2–); 3.39–3.42 (m, 4H, 2× –CH2–); 3.70–3.74 (m, 4H, 2× –CH2–); 4.15 (s, 2H, –CH2–); 6.46 (d, J = 5.3 Hz, 1H, Ar–H); 7.25 (dd, J = 2.2, 8.7 Hz, 1H, Ar–H); 7.28 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 7.31 (d, J = 2.2 Hz, 1H, Ar–H); 7.45 (dd, J = 2.0, 9.0 Hz, 1H, Ar–H); 7.68 (d, J = 8.8 Hz, 1H, Ar–H); 7.79 (d, J = 2.1 Hz, 1H, Ar–H); 8.16 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 8.27 (d, J = 9.0 Hz, 1H, Ar–H); 8.39 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 25.4, 27.6, 37.9, 40.6, 42.7, 47.4, 66.3, 99.1, 108.5, 117.7, 118.2, 120.3, 124.5, 124.7, 125.2, 127.7, 133.9, 134.5, 149.6, 150.5, 152.7, 155.6, 166.4, 167.9, 168.2, 174.1. HRMS calcd for C27H28ClN5O4 [M + H]+ 522.1863 found 522.1878.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-2-(5-morpholino-1,3-dioxoisoindolin-2-yl)acetamide (8c).
Yield 81%; yellow solid; mp 160–161 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.30–1.45 (m, 6H, 3× –CH2–); 1.64–1.67 (m, 2H, –CH2–); 3.04–3.09 (m, 2H, –CH2–); 3.23–3.27 (m, 2H, –CH2–); 3.36–3.39 (m, 4H, 2× –CH2–); 3.72–3.75 (m, 4H, 2× –CH2–); 4.12 (s, 2H, –CH2–); 6.45 (d, J = 5.4 Hz, 1H, Ar–H); 7.22 (dd, J = 2.4, 8.6 Hz, 1H, Ar–H); 7.27 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 7.32 (d, J = 2.3 Hz, 1H, Ar–H); 7.42 (dd, J = 2.3, 8.9 Hz, 1H, Ar–H); 7.66 (d, J = 8.6 Hz, 1H, Ar–H); 7.77 (d, J = 2.2 Hz, 1H, Ar–H); 8.15 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 8.26 (d, J = 9.0 Hz, 1H, Ar–H); 8.38 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 26.4, 26.7, 28.1, 29.4, 39.0, 42.7, 47.5, 48.9, 66.2, 99.0, 108.2, 117.9, 118.0, 120.1, 124.4, 124.5, 125.0, 127.9, 133.7, 134.7, 149.5, 150.5, 152.4, 155.7, 166.5, 167.8, 168.3, 174.2. HRMS calcd for C29H32ClN5O4 [M + H]+ 550.2176 found 550.2188.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-3-(5-morpholino-1,3-dioxoisoindolin-2-yl)propanamide (8d).
Yield 76%; yellow solid; mp 208–209 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.38 (t, J = 7.3 Hz, 2H, –CH2–); 3.20–3.28 (m, 8H, 4× –CH2–); 3.67–3.73 (m, 6H, 3× –CH2–); 6.44 (d, J = 5.6 Hz, 1H, Ar–H); 7.06 (dd, J = 2.3, 8.4 Hz, 1H, Ar–H); 7.16 (d, J = 2.2 Hz, 1H, Ar–H); 7.25 (t, J = 5.1 Hz, 1H, N–H-exchangeable with D2O); 7.36 (dd, J = 2.3, 9.0 Hz, 1H, Ar–H); 7.50 (d, J = 8.6 Hz, 1H, Ar–H); 7.73 (d, J = 2.3 Hz, 1H, Ar–H); 8.02 (d, J = 9.0 Hz, 1H, Ar–H); 8.24 (t, J = 5.5 Hz, 1H, N–H-exchangeable with D2O); 8.35 (d, J = 5.1 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 34.7, 34.8, 37.7, 42.8, 47.4, 66.2, 99.0, 108.0, 117.7, 117.8, 119.9, 124.2, 124.5, 124.7, 127.9, 133.8, 134.4, 149.4, 150.4, 152.3, 155.5, 168.0, 168.3, 170.9. HRMS calcd for C26H26ClN5O4 [M + H]+ 508.1707 found 508.1716.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-3-(5-morpholino-1,3-dioxoisoindolin-2-yl)propanamide (8e).
Yield 82%; Yellow solid; mp 158–159 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.52–1.57 (m, 2H, –CH2–); 1.61–1.67 (m, 2H, –CH2–); 2.36 (t, J = 7.1 Hz, 2H, –CH2–); 3.19–3.28 (m, 8H, 4× –CH2–); 3.65–3.71 (m, 6H, 3× –CH2–); 6.42 (d, J = 5.4 Hz, 1H, Ar–H); 7.04 (dd, J = 2.2, 8.7 Hz, 1H, Ar–H); 7.14 (d, J = 2.2 Hz, 1H, Ar–H); 7.23 (t, J = 5.3 Hz, 1H, N–H-exchangeable with D2O); 7.32 (dd, J = 2.2, 8.9 Hz, 1H, Ar–H); 7.51 (d, J = 8.7 Hz, 1H, Ar–H); 7.71 (d, J = 2.2 Hz, 1H, Ar–H); 8.01 (d, J = 9.0 Hz, 1H, Ar–H); 8.25 (t, J = 5.4 Hz, 1H, N–H-exchangeable with D2O); 8.33 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 25.5, 27.4, 34.6, 34.9, 37.9, 42.9, 47.7, 66.1, 99.1, 108.2, 117.5, 117.9, 119.4, 124.1, 124.6, 124.8, 127.7, 133.6, 134.2, 149.1, 150.2, 152.1, 155.6, 168.1, 168.2, 170.7. HRMS calcd for C28H30ClN5O4 [M + H]+ 536.2020 found 536.2009.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-3-(5-morpholino-1,3-dioxoisoindolin-2-yl)propanamide (8f).
Yield 78%; yellow solid; mp 126–127 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.31–1.46 (m, 6H, 3× –CH2–); 1.53–1.56 (m, 2H, –CH2–); 2.35 (t, J = 7.1 Hz, 2H, –CH2–); 3.05–3.09 (m, 2H, –CH2–); 3.23–3.27 (m, 2H, –CH2–); 3.35–3.39 (m, 4H, 2× –CH2–); 3.66–3.73 (m, 6H, 3× –CH2–); 6.41 (d, J = 5.3 Hz, 1H, Ar–H); 7.03 (dd, J = 2.1, 8.6 Hz, 1H, Ar–H); 7.15 (d, J = 2.1 Hz, 1H, Ar–H); 7.25 (t, J = 5.2 Hz, 1H, N–H-exchangeable with D2O); 7.33 (dd, J = 2.0, 8.9 Hz, 1H, Ar–H); 7.53 (d, J = 8.8 Hz, 1H, Ar–H); 7.73 (d, J = 2.1 Hz, 1H, Ar–H); 8.03 (d, J = 9.0 Hz, 1H, Ar–H); 8.26 (t, J = 5.3 Hz, 1H, N–H-exchangeable with D2O); 8.34 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 26.5, 26.9, 28.1, 29.5, 34.6, 38.2, 40.1, 42.8, 47.6, 66.2, 99.2, 108.1, 117.6, 117.8, 119.3, 124.2, 124.7, 124.8, 127.6, 133.7, 134.1, 149.3, 150.1, 152.4, 155.5, 168.1, 168.3, 170.5 HRMS calcd for C30H34ClN5O4 [M + H]+ 564.2333 found 564.2326.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-2-(5-(diethylamino)-1,3-dioxoisoindolin-2-yl)acetamide (8g).
Yield 79%; yellow solid; mp 197–198 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.10 (t, 6H, 2× –CH3); 3.22–3.27 (m, 4H, 2× –CH2–); 3.36–3.41 (m, 4H, 2× –CH2–); 4.11 (s, 2H, –CH2–); 6.52 (d, J = 5.5 Hz, 1H, Ar–H); 6.91 (d, J = 8.8 Hz, 1H, Ar–H); 6.97 (s, 1H, Ar–H), 7.46–7.50 (m, 2H, Ar–H, NH-exchangeable with D2O); 7.56 (d, J = 8.6 Hz, 1H, Ar–H); 7.77 (s, 1H, Ar–H), 8.16 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 8.28 (d, J = 8.9 Hz, 1H, Ar–H); 8.38 (d, J = 5.6 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 12.4, 37.6, 40.8, 42.6, 44.7, 99.0, 105.3, 114.7, 116.2, 117.8, 124.4, 124.6, 125.5, 127.2, 127.7, 134.1, 135.5, 150.7, 151.7, 152.2, 166.6, 167.6, 168.9 HRMS calcd for C25H26ClN5O3 [M + H]+ 480.1758 found 480.1743.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-2-(5-(diethylamino)-1,3-dioxoisoindolin-2-yl)acetamide (8h).
Yield 78%; yellow solid; mp 173–174 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.13 (t, 6H, 2× –CH3); 1.50–1.57 (m, 2H, –CH2–); 1.62–1.68 (m, 2H, –CH2–); 3.10–3.14 (m, 2H, –CH2–); 3.27–3.31 (m, 2H, 2× –CH2–); 3.39–3.43 (m, 4H, 2× –CH2–); 4.10 (s, 2H, –CH2–); 6.51 (d, J = 5.6 Hz, 1H, Ar–H); 6.92 (d, J = 8.7 Hz, 1H, Ar–H); 6.99 (s, 1H, Ar–H), 7.44–7.50 (m, 2H, Ar–H + NH-exchangeable with D2O); 7.59 (d, J = 8.5 Hz, 1H, Ar–H); 7.78 (s, 1H, Ar–H), 8.18 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 8.29 (d, J = 9.0 Hz, 1H, Ar–H); 8.39 (d, J = 5.7 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 12.5, 25.5, 27.1, 38.8, 41.5, 42.5, 44.8, 99.1, 105.2, 114.8, 116.3, 117.7, 124.5, 124.6, 125.4, 127.1, 127.4, 134.2, 135.3, 150.9, 151.6, 152.3, 166.7, 167.9, 168.6 HRMS calcd for C27H30ClN5O3 [M + H]+ 508.2071 found 508.2079.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-2-(5-(diethylamino)-1,3-dioxoisoindolin-2-yl)acetamide (8i).
Yield 79%; yellow solid; mp 158–159 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.12 (t, 6H, 2× –CH3); 1.32–1.44 (m, 6H, 3× –CH2–); 1.63–1.67 (m, 2H, –CH2–); 3.07–3.10 (m, 2H, –CH2–); 3.25–3.30 (m, 2H, 2× –CH2–); 3.37–3.41 (m, 4H, 2× –CH2–); 4.11 (s, 2H, –CH2–); 6.50 (d, J = 5.4 Hz, 1H, Ar–H); 6.90 (dd, J = 2.2, 8.8 Hz, 1H, Ar–H); 6.98 (d, J = 2.3 Hz, 1H, Ar–H), 7.43–7.50 (m, 2H, Ar–H + NH-exchangeable with D2O); 7.54 (d, J = 8.6 Hz, 1H, Ar–H); 7.75 (d, J = 2.2 Hz 1H, Ar–H), 8.20 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 8.27 (d, J = 9.0 Hz, 1H, Ar–H); 8.37 (d, J = 5.4 Hz, 1H, Ar–H);13C NMR (100 MHz, DMSO-d6) δ 12.4, 26.3, 26.9, 28.2, 29.5, 39.2, 41.3, 42.9, 44.6, 99.2, 105.1, 114.8, 116.3, 117.6, 124.7, 124.9, 125.4, 127.3, 127.7, 134.3, 135.3, 150.8, 151.6, 152.4, 166.6, 167.8, 168.7 HRMS calcd for C29H34ClN5O3 [M + H]+ 536.2384 found 536.2371.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-3-(5-(diethylamino)-1,3-dioxoisoindolin-2-yl)propanamide (8j).
Yield 70%; yellow solid; mp 189–190 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.05 (t, J = 7.0 Hz, 6H, 2× –CH3); 2.37 (t, J = 7.3 Hz, 2H, –CH2); 3.22–3.26 (m, 4H, 2× –CH2–); 3.38–3.42 (m, 4H, 2× –CH2–); 3.69 (t, J = 7.3 Hz, 2H, –CH3); 6.45 (d, J = 5.4 Hz, 1H, Ar–H); 6.76 (dd, J = 2.6, 8.8 Hz, 1H, Ar–H); 6.85 (d, J = 2.6 Hz, 1H, Ar–H), 7.29 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 7.37 (dd, J = 2.2, 9.0 Hz, 1H, Ar–H); 7.44 (d, J = 8.6 Hz, 1H, Ar–H); 7.73 (d, J = 2.4 Hz 1H, Ar–H), 8.05 (d, J = 9.0 Hz, 1H, Ar–H); 8.23 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 8.35 (d, J = 5.0 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 12.6, 34.6, 37.7, 41.1, 42.7, 44.8, 99.0, 105.0, 114.6, 116.2, 117.8, 124.3, 124.5, 124.6, 125.2, 127.8, 133.9, 135.0, 149.3, 150.5, 152.2, 168.1, 168.6, 170.9 HRMS calcd for C26H28ClN5O3 [M + H]+ 494.1914 found 494.1902.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-3-(5-(diethylamino)-1,3-dioxoisoindolin-2-yl)propanamide (8k).
Yield 72%; yellow solid; mp 161–162 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.04 (t, J = 7.1 Hz, 6H, 2× –CH3); 1.52–1.57 (m, 2H, –CH2–); 1.60–1.67 (m, 2H, –CH2–); 2.35 (t, J = 7.2 Hz, 2H, –CH3); 3.11–3.15 (m, 2H, –CH2–); 3.25–3.30 (m, 2H, –CH2–); 3.37–3.41 (m, 4H, 2× –CH2–); 3.67 (t, J = 7.2 Hz, 2H, –CH3); 6.43 (d, J = 5.3 Hz, 1H, Ar–H); 6.77 (dd, J = 2.4, 8.7 Hz, 1H, Ar–H); 6.84 (d, J = 2.4 Hz, 1H, Ar–H), 7.26 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 7.37 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.45 (d, J = 8.7 Hz, 1H, Ar–H); 7.75 (d, J = 2.2 Hz 1H, Ar–H), 8.07 (d, J = 9.0 Hz, 1H, Ar–H); 8.25 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 8.36 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 12.6, 25.4, 27.5, 34.6, 37.7, 39.7, 42.7, 44.7, 99.1, 105.2, 114.3, 116.1, 117.8, 124.2, 124.5, 124.7, 125.4, 127.9, 133.7, 135.1, 149.3, 150.6, 152.4, 168.1, 168.5, 170.7 HRMS calcd for C28H32ClN5O3 [M + H]+ 522.2227 found 522.2239.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-3-(5-(diethylamino)-1,3-dioxoisoindolin-2-yl)propanamide (8l).
Yield 68%; yellow solid; mp 142–143 °C; 1H NMR (400 MHz, DMSO-d6) δ 1.08 (t, J = 7.3 Hz, 6H, 2× –CH3); 1.35–1.45 (m, 6H, 3× –CH2–); 1.61–1.66 (m, 2H, –CH2–); 2.37 (t, J = 7.2 Hz, 2H, –CH3); 3.10–3.14 (m, 2H, –CH2–); 3.24–3.30 (m, 2H, –CH2–); 3.36–3.41 (m, 4H, 2× –CH2–); 3.68 (t, J = 7.2 Hz, 2H, –CH3); 6.42 (d, J = 5.2 Hz, 1H, Ar–H); 6.75 (dd, J = 2.2, 8.6 Hz, 1H, Ar–H); 6.85 (d, J = 2.4 Hz, 1H, Ar–H), 7.26 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 7.37 (dd, J = 2.0, 9.0 Hz, 1H, Ar–H); 7.47 (d, J = 8.7 Hz, 1H, Ar–H); 7.77 (d, J = 2.2 Hz 1H, Ar–H), 8.06 (d, J = 9.0 Hz, 1H, Ar–H); 8.26 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 8.38 (d, J = 5.2 Hz, 1H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 12.6, 26.4, 26.9, 28.5, 29.8, 34.6, 39.5, 41.1, 42.7, 44.5, 99.1, 105.3, 114.4, 116.1, 117.9, 124.2, 124.6, 124.8, 125.4, 127.8, 133.8, 135.2, 149.4, 150.7, 152.5, 168.1, 168.7, 170.6 HRMS calcd for C30H36ClN5O3 [M + H]+ 550.2540 found 550.2521.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-2-(5-(4-(2-hydroxyethyl)piperazin-1-yl)-1,3-dioxoisoindolin-2-yl)acetamide (8m).
Yield 75%; yellow solid; mp 218–219 °C; 1H NMR (500 MHz, DMSO-d6) δ 2.45 (t, J = 6.1 Hz, 2H, –CH2–); 2.54 (t, J = 4.8 Hz, 4H, 2× –CH2–); 3.11–3.15 (m, 2H, –CH2–); 3.26–3.29 (m, 2H, –CH2–); 3.42 (t, J = 4.5 Hz, 4H, 2× –CH2–); 3.53 (t, J = 6.1 Hz, 2H, –CH2–); 4.10 (s, 2H, –CH2–); 4.48 (s, 1H, –OH); 6.47 (d, J = 5.4 Hz, 1H, Ar–H); 7.21 (dd, J = 1.5, 8.6 Hz, 1H, Ar–H); 7.30 (d, J = 1.4 Hz, 1H, Ar–H); 7.35 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 7.45 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.66 (d, J = 8.6 Hz, 1H, Ar–H); 7.78 (d, J = 2.0 Hz, 1H, Ar–H); 8.18 (t, J = 5.7 Hz, 1H, NH-exchangeable with D2O); 8.29 (d, J = 9.0 Hz, 1H, Ar–H); 8.39 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 38.7, 42.4, 47.5, 49.1, 53.3, 59.1, 60.6, 99.0, 108.1, 117.3, 117.8, 119.4, 124.3, 124.6, 125.1, 127.9, 133.7, 134.7, 149.5, 150.6, 152.2, 155.5, 166.7, 167.8, 168.2 HRMS calcd for C27H29ClN6O4 [M + H]+ 537.1972 found 537.1961.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-2-(5-(4-(2-hydroxyethyl)piperazin-1-yl)-1,3-dioxoisoindolin-2-yl)acetamide (8n).
Yield 83%; yellow solid; mp 151–152 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.50–1.55 (m, 2H, –CH2–); 1.62–1.68 (m, 2H, –CH2–); 2.44 (t, J = 6.1 Hz 2H, –CH2–); 2.55 (t, J = 4.9 Hz, 4H, 2× –CH2–); 3.10–3.14 (m, 2H, –CH2–); 3.25–3.29 (m, 2H, –CH2–); 3.41 (t, J = 4.5 Hz, 4H, 2× –CH2–); 3.54 (t, J = 6.1 Hz, 2H, –CH2–); 4.11 (s, 2H, –CH2–); 4.49 (s, 1H, –OH); 6.48 (d, J = 5.5 Hz, 1H, Ar–H); 7.22 (dd, J = 1.5, 8.5 Hz, 1H, Ar–H); 7.31 (d, J = 1.4 Hz, 1H, Ar–H); 7.34 (t, J = 5.6 Hz, 1H, NH-exchangeable with D2O); 7.43 (dd, J = 2.2, 9.0 Hz, 1H, Ar–H); 7.65 (d, J = 8.5 Hz, 1H, Ar–H); 7.77 (d, J = 2.0 Hz, 1H, Ar–H); 8.19 (t, J = 5.7 Hz, 1H, NH-exchangeable with D2O); 8.27 (d, J = 9.0 Hz, 1H, Ar–H); 8.38 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 25.5, 27.1, 38.8, 42.5, 47.3, 49.0, 53.2, 59.0, 60.5, 99.1, 108.2, 117.8, 117.9, 119.4, 124.4, 124.5, 125.0, 127.8, 133.8, 134.7, 149.4, 150.5, 152.2, 155.5, 166.6, 167.8, 168.3 HRMS calcd for C29H33ClN6O4 [M + H]+ 565.2285 found 565.2268.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-2-(5-(4-(2-hydroxyethyl)piperazin-1-yl)-1,3-dioxoisoindolin-2-yl)acetamide (8o).
Yield 75%; yellow solid; mp 118–119 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.31–1.45 (m, 6H, 3× –CH2–); 1.62–1.67 (m, 2H, –CH2–); 2.46 (t, J = 6.2 Hz, 2H, –CH2–); 2.54 (t, J = 4.8 Hz, 4H, 2× –CH2–); 3.07–3.11 (m, 2H, –CH2–); 3.20–3.27 (m, 2H, –CH2–); 3.40 (t, J = 4.7 Hz, 4H, 2× –CH2–); 3.55 (t, J = 6.2 Hz, 2H, –CH2–); 4.10 (s, 2H, –CH2–); 4.48 (s, 1H, –OH); 6.47 (d, J = 5.4 Hz, 1H, Ar–H); 7.20 (dd, J = 1.6, 8.6 Hz, 1H, Ar–H); 7.30 (d, J = 1.5 Hz, 1H, Ar–H); 7.34 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 7.44 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.64 (d, J = 8.4 Hz, 1H, Ar–H); 7.75 (d, J = 2.0 Hz, 1H, Ar–H); 8.16 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 8.25 (d, J = 9.0 Hz, 1H, Ar–H); 8.36 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 26.1, 26.8, 28.1, 29.4, 39.5, 42.9, 47.5, 49.1, 53.5, 59.1, 60.4, 99.0, 108.1, 117.9, 118.1, 119.3, 124.5, 124.8, 125.1, 127.9, 133.7, 134.6, 149.3, 150.6, 152.1, 155.7, 166.6, 167.7, 168.2 HRMS calcd for C31H37ClN6O4 [M + H]+ 593.2598 found 593.2604.
N-(2-((7-Chloroquinolin-4-yl)amino)ethyl)-3-(5-(4-(2-hydroxyethyl)piperazin-1-yl)-1,3-dioxoisoindolin-2-yl)propanamide (8p).
Yield 69%; yellow solid; mp 211–212 °C; 1H NMR (500 MHz, DMSO-d6) δ 2.42–2.45 (m, 4H, 2× –CH2–); 2.53 (t, J = 5.1 Hz, 4H, 2× –CH2–); 3.26–3.31 (m, 4H, 2× –CH2–); 3.33–3.35 (m, 4H, 2× –CH2–); 3.52–3.55 (m, 2H, –CH2–); 3.76 (t, J = 7.1 Hz, 2H, –CH2–); 4.47 (s, 1H, OH); 6.49 (d, J = 5.4 Hz, 1H, Ar–H); 7.11 (dd, J = 2.3, 8.5 Hz, 1H, Ar–H); 7.19 (d, J = 2.3 Hz, 1H, Ar–H); 7.28 (t, J = 5.2 Hz, 1H, NH-exchangeable with D2O); 7.40 (dd, J = 2.2, 9.0 Hz, 1H, Ar–H); 7.53 (d, J = 8.4 Hz, 1H, Ar–H); 7.77 (d, J = 2.3 Hz, 1H, Ar–H); 8.08 (d, J = 8.9 Hz, 1H, Ar–H); 8.25 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 8.39 (d, J = 5.4 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 34.7, 34.8, 37.7, 42.7, 47.2, 53.2, 59.0, 60.5, 99.0, 107.9, 117.7, 117.8, 119.2, 124.2, 124.5, 124.8, 127.9, 133.8, 134.5, 149.4, 150.4, 152.3, 153.3, 168.0, 168.4, 170.9. HRMS calcd for C28H31ClN6O4 [M + H]+ 551.2129 found 551.2141.
N-(4-((7-Chloroquinolin-4-yl)amino)butyl)-3-(5-(4-(2-hydroxyethyl)piperazin-1-yl)-1,3-dioxoisoindolin-2-yl)propanamide (8q).
Yield 68%; yellow solid; mp 162–163 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.50–1.55 (m, 2H, –CH2–); 1.62–1.68 (m, 2H, –CH2–); 2.41–2.47 (m, 4H, 2× –CH2–); 2.55 (t, J = 5.0 Hz, 4H, 2× –CH2–); 3.13–3.16 (m, 2H, –CH2–); 3.27–3.31 (m, 2H, –CH2–); 3.41 (t, J = 4.5 Hz, 4H, 2× –CH2–); 3.54 (t, J = 6.1 Hz, 2H, –CH2–); 3.75 (t, J = 7.1 Hz, 2H, –CH2–); 4.48 (s, 1H, OH); 6.47 (d, J = 5.5 Hz, 1H, Ar–H); 7.10 (dd, J = 2.2, 8.6 Hz, 1H, Ar–H); 7.18 (d, J = 2.2 Hz, 1H, Ar–H); 7.27 (t, J = 5.3 Hz, 1H, NH-exchangeable with D2O); 7.41 (dd, J = 2.1, 9.0 Hz, 1H, Ar–H); 7.52 (d, J = 8.5 Hz, 1H, Ar–H); 7.78 (d, J = 2.2 Hz, 1H, Ar–H); 8.05 (d, J = 9.0 Hz, 1H, Ar–H); 8.27 (t, J = 5.4 Hz, 1H, NH-exchangeable with D2O); 8.40 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 25.4, 27.9, 34.6, 37.9, 39.1, 42.7, 47.8, 53.1, 59.1, 60.6, 99.1, 107.8, 117.4, 117.9, 119.1, 124.3, 124.5, 124.9, 127.8, 133.9, 134.6, 149.6, 150.3, 152.5, 153.5, 168.1, 168.7, 170.6. HRMS calcd for C30H35ClN6O4 [M + H]+ 579.2442 found 579.2457.
N-(6-((7-Chloroquinolin-4-yl)amino)hexyl)-3-(5-(4-(2-hydroxyethyl)piperazin-1-yl)-1,3-dioxoisoindolin-2-yl)propanamide (8r).
Yield 64%; yellow solid; mp 102–103 °C; 1H NMR (500 MHz, DMSO-d6) δ 1.32–1.44 (m, 6H, 3× –CH2–); 1.63–1.68 (m, 2H, –CH2–); 2.42–2.48 (m, 4H, 2× –CH2–); 2.54 (t, J = 5.0 Hz, 4H, 2× –CH2–); 3.11–3.15 (m, 2H, –CH2–); 3.24–3.30 (m, 2H, –CH2–); 3.43 (t, J = 4.8 Hz, 4H, 2× –CH2–); 3.57 (t, J = 6.1 Hz, 2H, –CH2–); 3.75 (t, J = 7.1 Hz, 2H, –CH2–); 4.47 (s, 1H, OH); 6.45 (d, J = 5.4 Hz, 1H, Ar–H); 7.09 (dd, J = 2.1, 8.7 Hz, 1H, Ar–H); 7.16 (d, J = 2.1 Hz, 1H, Ar–H); 7.28 (t, J = 5.1 Hz, 1H, NH-exchangeable with D2O); 7.40 (dd, J = 2.0, 9.0 Hz, 1H, Ar–H); 7.51 (d, J = 8.4 Hz, 1H, Ar–H); 7.79 (d, J = 2.1 Hz, 1H, Ar–H); 8.07 (d, J = 9.0 Hz, 1H, Ar–H); 8.29 (t, J = 5.5 Hz, 1H, NH-exchangeable with D2O); 8.41 (d, J = 5.3 Hz, 1H, Ar–H); 13C NMR (125 MHz, DMSO-d6) δ 26.3, 26.9, 28.4, 29.6, 34.5, 39.7, 42.8, 47.4, 49.3, 53.7, 59.3, 60.6, 99.0, 107.7, 117.5, 117.9, 119.3, 124.4, 124.8, 124.8, 127.9, 133.8, 134.7, 149.5, 150.5, 152.8, 153.8, 168.2, 168.9, 170.5. HRMS calcd for C32H39ClN6O4 [M + H]+ 607.2755 found 607.2769.
Materials and methods
Bacterial strains and growth conditions
M. tuberculosis mc26230 (ref. 29) was grown at 37 °C in Middlebrook 7H9 supplemented with oleic-albumin-dextrose-catalase enrichment (OADC) and 109 μM of pantothenic acid (complete 7H9).
Drug susceptibility testing
The vulnerability of M. tuberculosis mc26230 to the synthesized compounds was determined as reported previously.30 Briefly, an exponentially growing culture was diluted in complete 7H9 to and OD600 = 0.01. The bacteria were then seeded in 100 μL volumes in all the wells of a 96-well plate except the first column of wells which contained 200 μL of bacterial suspension. Compounds (stock concentration 10 mg mL−1) were directly added to the wells of the first column so as to achieve a concentration of 200 μg mL−1 (or 100 μg mL−1 in cases where precipitation at the higher concentration was observed). Two-fold serial dilutions were then carried out by transferring 100 μL of bacterial suspension from the first column of wells to the second column, mixing, and repeating this procedure for each consecutive column. The plates were then placed in sealed plastic bags and incubated at 37 °C. After 7 days of incubation, plates were visually inspected to determine the MIC, which was defined as the minimal concentration of compound at which no growth of bacteria was observed. Drug susceptibility testing was completed twice, with each compound tested in duplicate. Isoniazid was included as a positive control.
Cytotoxicity assay
Cell viability was determined using Vero cells (ATCC, Sigma, Germany) grown in RPMI medium (Gibco, USA), supplemented with 10% decomplemented fetal calf serum, under a 5% CO2 atmosphere. Cells were seeded in 96-well plates at a density of 2 × 104 cells per well in 160 μL medium and incubated overnight at 37 °C to allow cells to adhere. Compounds (dissolved in DMSO) were freshly diluted to appropriate concentrations in RPMI, so as to allow addition of 20 μL volumes of the diluted compounds to the cells that resulted in final compound concentrations ranging from 100 μg mL−1 to 0.78 μg mL−1. The maximum final concentration of DMSO was 1% (v/v) and no cytotoxic effect of DMSO was observed at this concentration. After 24 h incubation at 37 °C, 20 μL of 1 mg mL−1 resazurin (Sigma, Germany) was added to each well and the cells were incubated for an additional 3 hours at 37 °C. Fluorescence was measured in a Polarstar Omega fluorometer using appropriate filters (540 nm excitation and 590 nm emission wave length). Percentage survival was determined by dividing fluorescence values obtained in the compound containing wells by values obtained for control wells containing cells incubated with a dilution series of DMSO and multiplying this value by 100. SDS (20%) was included as a positive control. Cytotoxic evaluation was completed twice, with each compound tested in duplicate. The IC50 is defined as the lowest concentration of compound tested at which exactly 50% cell viability was observed and was calculated using a non-linear regression curve using Graphpad Prism 5. The SI values were determined as a function of IC50/MIC99.
Conflicts of interest
The authors declare no conflict of interest.
Abbreviations
| MIC990 | 99% minimum inhibitory concentration |
| MW | microwave |
| +SAR | Structure activity relationship |
| NMP |
N-Methyl-2-pyrrolidone |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| SI value | Selectivity index |
| IC50 | 50% inhibitory concentration |
Acknowledgements
Council of Scientific and Industrial Research (CSIR), New Delhi, India was acknowledged by AR for CSIR-JRF Fellowship (A.R.) with Ref. No. 09/254(0269)/2017-EMR-1. Science and Engineering Research Board (SERB), New Delhi was acknowledged by VK for financial assistance with grant no. EMR/2015/001687. LK acknowledges the support by the Fondation pour la Recherche Médicale (FRM) (DEQ20150331719) and the Labex EpiGenMed under the program « Investissements d'avenir » (ANR-10-LABX-12-01).
References
- S. Chetty, M. Ramesh, A. S. Pillay and M. E. S. Soliman, Bioorg. Med. Chem. Lett., 2017, 27, 370–386 CrossRef CAS.
-
World Health Organization (WHO), Global tuberculosis report, 2016, available from: http://www.apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1, 2016, accessed 3 October 2017 Search PubMed.
-
Revised National Tuberculosis Control Program, Training manual on intensified TB/HIV package, Ministry of Health and Family Welfare, New Delhi, 2017 Search PubMed.
- X. Lu, J. Tang, S. Cui, B. Wan, S. G. Franzblauc, T. Zhang, X. Zhang and K. Ding, Eur. J. Med. Chem., 2017, 125, 41 CrossRef CAS.
- WHO Multidrug and extensively drug-resistant TB 2010 Global report on surveillance and response (http://www.apps.who.int/iris/bitstream/10665/44286/1/9789241599191_eng.pdf).
- H. Janmanchi, A. Raju, M. S. Degani, M. K. Ray and M. G. R. Rajan, S. Afr. J. Bot., 2017, 113, 421 CrossRef CAS.
- A. H. Diacon, A. Pym, M. Grobusch, R. Patientia, R. Rustomjee, L. Page-Shipp, C. Pistorius, R. Krause, M. Bogoshi, G. Churchyard, A. Venter, J. Allen, J. C. Palomino, T. De Marez, R. P. van Heeswijk, N. Lounis, P. Meyvisch, J. Verbeeck, W. Parys, K. de Beule, K. Andries and D. F. Mc Neeley, N. Engl. J. Med., 2009, 360, 2397 CrossRef CAS.
- N. Veziris, M. Ibrahim, N. Lounis, T. Andries and V. Jarlier, PLoS One, 2011, 6, 17556 CrossRef.
- J. Cohen, Science, 2013, 339, 130 CrossRef CAS PubMed.
- A. H. Diacon, A. Pym, M. P. Grobusch, J. M. de los Rios, E. Gotuzzo, I. Vasilyeva, V. Leimane, K. Andries, N. Bakare, T. De Marez, M. Haxaire-Theeuwes, N. Lounis, P. Meyvisch, E. De Paepe, R. P. G. van Heeswijk and B. Dannemann, N. Engl. J. Med., 2014, 371, 723 CrossRef.
- S. Umamatheswari and C. Sankar, Bioorg. Med. Chem. Lett., 2017, 27, 695 CrossRef CAS.
- S. Jayaprakash, Y. Iso, B. Wan, S. G. Franzblau and A. P. Kozikowski, ChemMedChem, 2006, 1, 593 CrossRef CAS.
- L. E. Bermudez, P. Kolonoski, M. Wu, P. A. Aralar, C. B. Inderlied and L. S. Young, Antimicrob. Agents Chemother., 1999, 43, 1870 CrossRef CAS.
- L. E. Bermudez, P. Kolonoski, L. E. Seitz, M. Petrofsky, R. Reynolds and M. Wu, Antimicrob. Agents Chemother., 2004, 48, 3556 CrossRef CAS.
- L. E. Bermudez, P. Kolonoski, M. Petrofsky, M. Wu, C. B. Inderlied and L. S. Young, J. Infect. Dis., 2003, 187, 1977 CrossRef CAS.
- J. Mao, H. Yuan, Y. Wang, B. Wan, D. Pak, R. He and S. G. Franzblau, Bioorg. Med. Chem. Lett., 2010, 20, 1263 CrossRef CAS.
- J. Mao, Y. Wang, B. Wan, A. P. Kozikowski and S. G. Franzblau, ChemMedChem, 2007, 2, 1624 CrossRef CAS PubMed.
- U. Sharma, P. Kumar and B. Kumar, Mini-Rev. Med. Chem., 2010, 10, 678 CrossRef CAS PubMed.
- S. M. Capitosti, T. P. Hansen and M. L. Brown, Bioorg. Med. Chem. Lett., 2004, 12, 327 CrossRef CAS.
- S. G. Stewart, C. J. Braun, S. L. Ng, M. E. Polomska, M. Karimi and L. J. Abraham, Bioorg. Med. Chem., 2010, 18, 650 CrossRef CAS.
- S. H. L. Kok, R. Gambari, C. H. Chu, M. C. W. Yuen, E. Lin, R. S. M. Wong, F. Y. Lau, G. Y. M. Cheng, W. S. Lam, S. H. Chan, K. H. Lam, C. H. Cheng, P. B. S. Lai, M. W. Y. Yu, F. Cheung, J. C. O. Tang and A. S. C. Chan, Bioorg. Med. Chem., 2008, 16, 3626 CrossRef CAS.
- S. M. Sami, R. T. Dorr, D. S. Alberts, A. M. Solyom and W. A. Remers, J. Med. Chem., 2000, 43, 3067 CrossRef CAS.
- R. Dahlbom, B. Karlen, R. George and D. J. Jenden, J. Med. Chem., 1966, 9, 843–846 CrossRef CAS.
- H. Akgun, I. Karamelekoglu, B. Berk, I. Kurnaz, G. Saribiyik, S. Oktem and T. Kocagoz, Bioorg. Med. Chem., 2012, 20, 4149 CrossRef CAS.
-
(a) J. L. Santos, P. R. Yamasaki, C. M. Chin, C. H. Takashi, F. R. Pavan and C. Q. F. Leite, Bioorg. Med. Chem., 2009, 17, 3795 CrossRef CAS;
(b) A. Kamal, A. HariBabu, A. V. Ramana, R. Sinha, J. S. Yadava and S. K. Arorab, Bioorg. Med. Chem. Lett., 2005, 15, 1923 CrossRef CAS.
- A. Rani, A. Viljoen, Sumanjit, L. Kremer and V. Kumar, ChemistrySelect, 2017, 2, 10782 CrossRef CAS.
-
(a) A. Singh, A. Viljoen, L. Kremer and V. Kumar, Future Med. Chem., 2017, 9, 1701 CrossRef CAS;
(b) A. Singh, C. Biot, A. Viljoen, C. Dupont, L. Kremer, K. Kumar and V. Kumar, Chem. Biol. Drug Des., 2017, 89, 856 CrossRef CAS PubMed;
(c) A. Singh, J. Gut, P. J. Rosenthal and V. Kumar, Eur. J. Med. Chem., 2017, 5, 269 CrossRef PubMed;
(d) S. Kumar, A. Saini, J. Gut, P. J. Rosenthal, R. Raj and V. Kumar, Eur. J. Med. Chem., 2017, 138, 993 CrossRef CAS.
- D. Di, F. M. Krogstad, L. D. Byers and D. J. Krogstad, J. Med. Chem., 1998, 41, 4918 CrossRef.
- V. K. Sambandamurthy, S. C. Derrick, T. Hsu, B. Chen, M. H. Larsen, K. V. Jalapathy, M. Chen, J. Kim, S. A. Porcelli, J. Chan, S. L. Morris and W. R. Jacobs Jr, Vaccine, 2006, 24, 6309 CrossRef CAS.
- J. Ollinger, M. A. Bailey, G. C. Moraski, A. Casey, S. Florio, T. Alling, M. J. Miller and T. Parish, PLoS One, 2013, 8, e60531 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Scanned 1H and 13C NMR spectra of few representative compounds viz. 4c, 4d, 4f, 4k, 4m, 4o, 4s, 7a, 7c, 7e, 7i, 7n, 7q, 8a, 8c, 8d, 8j, 8n and 8p are provided in the electronic supplementary information. See DOI: 10.1039/c8ra10532d |
|
| This journal is © The Royal Society of Chemistry 2019 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths among HIV-positive people, mostly in developing countries.2 According to the Revised National Tuberculosis Control Program (RNTCP), India accounts for one fourth of the global TB burden with 1.75 million TB patients, including data from both the public and private health sectors, while 33
000 deaths among HIV-positive people, mostly in developing countries.2 According to the Revised National Tuberculosis Control Program (RNTCP), India accounts for one fourth of the global TB burden with 1.75 million TB patients, including data from both the public and private health sectors, while 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 drug-resistant TB patients are noted additionally.3 Increasing cases of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB all over the world is hindering current TB treatment,4 which includes the first-line drugs isoniazid (INH), rifampicin (RIF), pyrazinamide, ethambutol, and streptomycin, and the second line drugs, aminoglycosides, polypeptides, fluoroquinolones, thioamides, cycloserine and terizidone.5,6 The emergence of resistant strains warrants the urgent search for faster-acting, less harmful, more efficient drug candidates.
820 drug-resistant TB patients are noted additionally.3 Increasing cases of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB all over the world is hindering current TB treatment,4 which includes the first-line drugs isoniazid (INH), rifampicin (RIF), pyrazinamide, ethambutol, and streptomycin, and the second line drugs, aminoglycosides, polypeptides, fluoroquinolones, thioamides, cycloserine and terizidone.5,6 The emergence of resistant strains warrants the urgent search for faster-acting, less harmful, more efficient drug candidates.






