Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phosphine-promoted [4 + 3] annulation of allenoate with aziridines for synthesis of tetrahydroazepines: phosphine-dependent [3 + 3] and [4 + 3] pathways†
Honglei Liu‡
a,
Yan Lin‡a,
Yan Zhaoa,
Miaoren Xiaob,
Leijie Zhoua,
Qijun Wanga,
Cheng Zhang a,
Dongqi Wangb,
Ohyun Kwon
a,
Dongqi Wangb,
Ohyun Kwon *c and
Hongchao Guo
*c and
Hongchao Guo *a
*a
aDepartment of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: hchguo@cau.edu.cn
bInstitute of High Energy Physics, Chinese Academy of Science, 19B Yuquan Lu, Shijingshan District, Beijing 100049, P. R. China
cDepartment of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, USA. E-mail: ohyun@chem.ucla.edu
First published on 9th January 2019
Abstract
In this manuscript, phosphine-dependent [3 + 3] and [4 + 3] annulation reactions of allenoate with aziridines were disclosed. The alkyldiphenylphosphine-promoted [4 + 3] annulation of allenoate with aziridines has been achieved under mild conditions, providing biologically interesting functionalized tetrahydroazepines in moderate to excellent yield with moderate to excellent regioselectivity and diastereoselectivity.
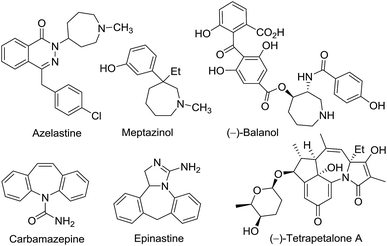
Nitrogen-containing heterocyclic compounds are widely present in biologically active natural products and synthetic pharmaceuticals. Among them, tetrahydropyridines which can be converted into pyridines and piperidines are intriguing synthetic targets due to their significant biological activities.1 In addition, azepines are widely found as the core structure in a large number of compounds that possess important pharmaceutical activities. The compounds containing the azepine moiety are important targets in synthetic and medicinal chemistry.2 Among these compounds (Fig. 1), azelastine is an effective and safe treatment agent for urticaria.3 Meptazinol is a new opioid-type analgesic with mixed agonist/antagonist properties.4 (−)-Balanol is a fungal metabolite with potent protein kinase C inhibitory properties.5 An anticonvulsant, carbamazepine, is known to show incidences of cutaneous adverse drug reactions including Stevens–Johnson syndrome, toxic epidermal necrolysis and drug-induced hypersensitivity syndrome.6 Epinastine is a potent antiallergic agent that not only has antihistaminic property but also provides antileukotriene, anti-PAF and antibradykinin activities.7 The tetracyclic natural product, (−)-tetrapetalone A is a novel lipoxygenase inhibitor from Streptomyces sp.8 Therefore, new synthetic methodologies for the synthesis of azepine derivatives have attracted much attention. Among various methods, the cycloaddition reactions are practical and efficient methods, and have been extensively investigated.
Nucleophilic phosphine-catalyzed cycloaddition reactions of allenoates have evolved as a very useful tool to access various complex ring systems of organic molecules.9,10 Since Lu and coworkers reported the first phosphine-catalyzed [3 + 2] cycloaddition of allenoates with electron-deficient alkenes in 1995,11 various types of cycloaddition reactions have been developed to afford different sizes of carbocycles or heterocycles.9 In spite of these advances, developing new cycloaddition reaction of allenoates is still of great significance to construct novel ring frameworks with functional groups.
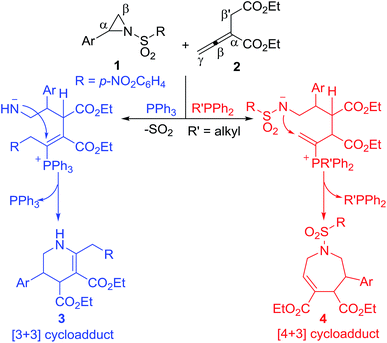
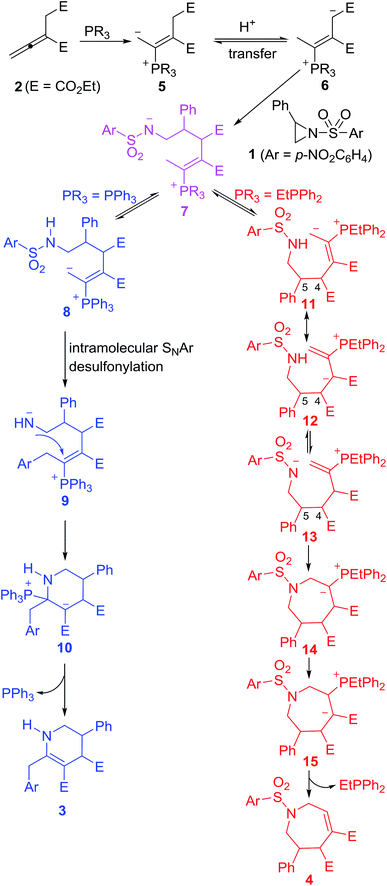
Aziridines are an important type of versatile building blocks for synthesis of diverse nitrogen-containing heterocyclic compounds and natural products.12 In the presence of Lewis acid or organocatalyst, aziridines may undergo a ring-opening reaction through C–N bond cleavage and work as a masked 1,3-dipole to react with various dipolarophiles, giving diverse cycloadducts. Many Lewis acid or organocatalyst-mediated cycloaddition reactions such as [3 + 2],13 [3 + 3],14 [6 + 3]15 and [8 + 3]16 cycloaddition reactions involving aziridines have been reported. In 2009, Kwon reported the first PPh3-promoted [3 + 3] annulation of aziridines with α-substituted allenoates to generate highly functionalized tetrahydropyridines by release of SO2.17a During the process, aziridines undergo a ring-opening reaction through the breakage of the C–N bond upon the attack of the zwitterionic adduct formed by the addition of PPh3 to an allenoate, and the resulting amide anion attacks the β-carbon of the allenoate after an intramolecular desulfonation to realize the [3 + 3] annulation (Scheme 1).17 The reaction is operationally simple and produces highly functionalized tetrahydropyridines in good to excellent yields with high levels of diastereoselectivity. In theory, however, the amide anion without the desulonation could attack the γ-carbon of the allenoates to result in a [4 + 3] annulation (Scheme 1).18 With this query in mind and our continuing interest in phosphine-catalyzed cycloaddition reactions,19 we herein report the first alkyldiphenylphosphine-promoted [4 + 3] annulation of aziridines with an allenoate to afford functionalized tetrahydroazepines under simple and mild reaction conditions (Scheme 1).
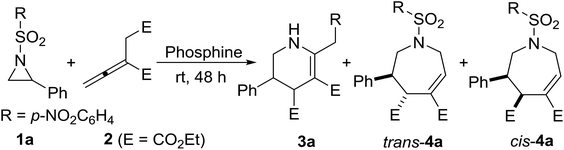
As shown in Scheme 1, in our previous work, in the presence of Ph3P, aziridines and α-substituted allenoates performed [3 + 3] annulation in dichloromethane at room temperature. Through revisiting the catalyst screening, we found that alkyldiphenyl-phosphines can reverse the regioselectivity, leading to [4 + 3] annulation, as shown in Table 1. The best result for [4 + 3] annulation of aziridine 1a and allenoate 2 was obtained when 1 equivalent of EtPPh2 was added, with 93% yield of the cycloadducts, 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 of regioselectivity and 81
8 of regioselectivity and 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 of diastereoselectivity (Table 1, entry 3). n-PrPPh2 is also an effective catalyst compared to PPh3, and gave similar result to that with EtPPh2 (entry 4). MePPh2, i-PrPPh2, n-BuPPh2, CyPPh2, DPPB, and DPPP gave good yield of cycloadducts with poor to moderate regioselectivity (entries 2, 5, 6, 8–11). t-BuPPh2 afforded much lower yield of cycloadducts although with excellent regio- and diastereoselectivity (100
19 of diastereoselectivity (Table 1, entry 3). n-PrPPh2 is also an effective catalyst compared to PPh3, and gave similar result to that with EtPPh2 (entry 4). MePPh2, i-PrPPh2, n-BuPPh2, CyPPh2, DPPB, and DPPP gave good yield of cycloadducts with poor to moderate regioselectivity (entries 2, 5, 6, 8–11). t-BuPPh2 afforded much lower yield of cycloadducts although with excellent regio- and diastereoselectivity (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (entry 7). Subsequently, the effect of solvents was evaluated with the model reaction using EtPPh2 as the catalyst. The results showed that the aprotic CH2Cl2 remained to be the best solvent, while MeOH gave excellent reaction selectivity but low yield of cycloadducts (entry 16). Other solvents, such as THF, CH3Cl, Cl(CH2)2Cl, and toluene afforded low to moderate yield of cyloadducts and lower reaction selectivity (entries 12–15). As such, CH2Cl2 was selected as the best solvent for the reaction. The relative configuration of the product 4a was determined by single-crystal X-ray analysis.20
0) (entry 7). Subsequently, the effect of solvents was evaluated with the model reaction using EtPPh2 as the catalyst. The results showed that the aprotic CH2Cl2 remained to be the best solvent, while MeOH gave excellent reaction selectivity but low yield of cycloadducts (entry 16). Other solvents, such as THF, CH3Cl, Cl(CH2)2Cl, and toluene afforded low to moderate yield of cyloadducts and lower reaction selectivity (entries 12–15). As such, CH2Cl2 was selected as the best solvent for the reaction. The relative configuration of the product 4a was determined by single-crystal X-ray analysis.20
| Entry | Phosphine (mol%) | Solvent | Yieldb (%) | 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ac 3ac |
dr (trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis) for 4ac cis) for 4ac |
|---|---|---|---|---|---|
| a Unless otherwise stated, all reactions were performed using 0.125 mmol of 1a and 0.150 mmol of 2 in 5 mL of CH2Cl2 at room temperature for 48 h.b Sum of the isolated yields of 3a and 4a.c Ratio of isolated yields.d React time is 72 h. DPPB: 1,4-bis(diphenylphosphino)butane; DPPP: 1,3-bis(diphenylphosphino)propane. | |||||
| 1 | PPh3 (100) | CH2Cl2 | 73 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
— |
| 2 | MePPh2 (100) | CH2Cl2 | 78 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
| 3 | EtPPh2 (100) | CH2Cl2 | 93 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 4 | n-PrPPh2 (100) | CH2Cl2 | 97 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | i-PrPPh2 (100) | CH2Cl2 | 35 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6 | n-BuPPh2 (100) | CH2Cl2 | 56 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 7 | t-BuPPh2 (100) | CH2Cl2 | 21 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 8 | CyPPh2 (100) | CH2Cl2 | 83 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 9 | DPPB (100) | CH2Cl2 | 35 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 10 | DPPB (50) | CH2Cl2 | 57 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 11 | DPPP (50) | CH2Cl2 | 48 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 12 | EtPPh2 (100) | Cl(CH2)2Cl | 43 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
| 13 | EtPPh2 (100) | CHCl3 | 44 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 14d | EtPPh2 (100) | Toluene | 42 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 15d | EtPPh2 (100) | THF | 66 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 16d | EtPPh2 (100) | MeOH | 32 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Under the optimized conditions, the annulation reactions of different aryl substituted aziridines with diethyl 2-vinylidenesuccinate were evaluated (Table 2). In most cases, regardless of the electronic nature of the substituent of the aryl group, using EtPPh2 or n-PrPPh2 as the catalyst, moderate to good yield and moderate to good selectivity of cycloadducts were obtained, and the yields are usually lower than that having the simple phenyl ring. The position of substituents on the benzene ring seems to have no significant influence on reactivity and selectivity. For example, substituents such as 4-MeC6H4 and 2,4,6-Me3C6H2 gave the desired products 4d and 4g in similar yields (entries 4 and 7). The annulation reaction also worked well with 2-naphthyl substituted aziridine (1n), affording the corresponding product in 58% yield (entry 14). Unfortunately, the alkyl substituent gave no desired product, due to the weak electrophilic properties of alkyl aziridines. All these products (4) are new compounds.
| Entry | Ar in 1 | R′PPh2 | T/°C | Yieldb (%) of 4 + 3 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c 3c |
4 | dr (trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis) for 4c cis) for 4c |
|---|---|---|---|---|---|---|---|
| a All of the reactions were performed using 0.125 mmol of 1a, 0.150 mmol of 2, and 0.125 mmol of catalyst in 5 mL of CH2Cl2 for 48 h.b Sum of the isolated yields of 3 and 4.c Ratio of isolated yields. | |||||||
| 1 | C6H5, 1a | EtPPh2 | 25 | 93 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
4a | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 2 | 2-MeC6H4, 1b | n-PrPPh2 | 25 | 65 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
4b | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 3 | 3-MeC6H4, 1c | n-PrPPh2 | 25 | 58 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
4c | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
| 4 | 4-MeC6H4, 1d | n-PrPPh2 | 20 | 72 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
4d | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 5 | 2,4-Me2C6H3, 1e | EtPPh2 | 25 | 96 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
4e | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
| 6 | 2,5-Me2C6H3, 1f | n-PrPPh2 | 20 | 46 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
4f | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 7 | 2,4,6-Me3C6H2, 1g | n-PrPPh2 | 25 | 77 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
4g | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 8 | 4-t-BuC6H4, 1h | n-PrPPh2 | 25 | 57 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
4h | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 9 | 2-FC6H4, 1i | n-PrPPh2 | 25 | 60 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
4i | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 10 | 3-FC6H4, 1j | n-PrPPh2 | 25 | 48 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
4j | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 11 | 4-FC6H4, 1k | n-PrPPh2 | 20 | 73 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
4k | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 12 | 2-ClC6H4, 1l | n-PrPPh2 | 25 | 78 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
4l | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 13 | 2-BrC6H4, 1m | n-PrPPh2 | 20 | 42 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
4m | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 14 | 2-Naphthyl, 1n | n-PrPPh2 | 25 | 58 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
4n | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
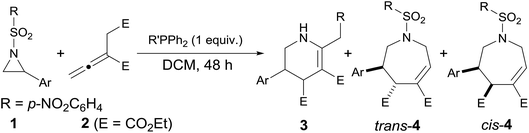
Two plausible pathways for the reactions of the aziridines 1 and the allenoate 2 are presented in Scheme 2. PPh3 and EtPPh2 or n-PrPPh2 were found to mainly lead to [3 + 3] and [4 + 3] annulations, respectively. The reaction starts with a nucleophilic addition of the catalyst to the allenoate 2. A subsequent proton transfer then occurs to neutralize the negative charge on the terminal γ-carbon atom of 5. The newly formed secondary carboanion 6 is nucleophilic, and may attack the electron-deficient C atom of the aziridine to give a zwitterionic intermediate 7. When PPh3 is used as catalyst, a proton transfer ensues to neutralize the negative charge on N atom and results in a primary carboanion 8. The formation of 8 may be followed by a desulfonylation step and the p-nitrophenyl group is migrated to the terminal γ-carbon, releasing a molecule of SO2 and leaving the negative charge on the N atom. A nucleophilic step then occurs to close the six-membered ring and the elimination of triphenylphosphine gives the [3 + 3] annulation product 3 with the catalyst being regenerated. Compared with PPh3, when alkyldiphenylphosphine is used as catalyst, the primary carboanion 11 isomerizes into intermediate 12, which performs a proton transfer from N atom to C atom to give the intermediate 13. The cyclization of 13 furnished the ylide 14, which undergoes a proton transfer to produce the intermediate 15. Through elimination of the phosphine, the β-phosphonium ester 15 was converted to the [4 + 3] annulation product 4. The carbon–carbon single bond between C4 and C5 in the intermediates 11, 12 and 13 might rotate, thus resulting in moderate diastereoselectivity.
Conclusions
In conclusion, we disclosed phosphine-dependent [3 + 3] and [4 + 3] annulations of allenoate with aziridines and developed the first phosphine-promoted [4 + 3] annulation involving aziridines. The reaction works efficiently under mild conditions to give functionalized tetrahydroazepines in moderate to excellent yield with moderate to excellent diastereoselectivity.Experimental
General methods
All reactions were performed under N2 atmospheres in oven-dried glassware with magnetic stirring. Unless otherwise stated, all reagents were purchased from commercial suppliers and used without further purification. All solvents were purified and dried according to standard methods prior to use. Organic solutions were concentrated under reduced pressure on a rotary evaporator or an oil pump. Reactions were monitored through thin layer chromatography (TLC) on silica gel-precoated glass plates (0.25 mm thickness, silica gel). Chromatograms were visualized by fluorescence quenching with UV light at 254 nm. Flash column chromatography was performed using flash silica gel (200–300 mesh). 1H and 13C NMR spectra were recorded in CDCl3 using a 300 MHz NMR instrument (referenced internally to Me4Si). Data for 13C NMR spectra are reported in terms of chemical shift. Melting points were determined on a melting point apparatus.Preparation of aziridines 1
The 2-aryl-1-(4-nitrobenzenesulfonyl) aziridines were prepared according to procedures described previously in the literature.17aPreparation of allenoate 2
The diethyl 2-vinylidenesuccinate 2 was prepared according to procedures described previously in the literature.17a,cGeneral procedure for the annulation of aziridines 1 and allenoate 2
An oven-dried 10 mL flask was charged with diphenyl-ethylphosphine or diphenyl-n-propylphosphine (0.125 mmol), the N-4-nitrobenzenesulfonyl-protected aziridine (0.125 mmol), and CH2Cl2 (5 mL) at room temperature. After adding diethyl 2-vinylidenesuccinate (0.15 mmol) to this solution, the mixture was stirred at room temperature for 48 h. The reaction mixture was concentrated and the residue purified through flash column chromatography (EtOAc/hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford the corresponding tetrahydroazepine product.
5) to afford the corresponding tetrahydroazepine product.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the NSFC (No. 21172253, 21372256, 21572264 and 21871293 to H. G, 21473206 to D. W.), Chinese Universities Scientific Fund (No. 2018TC052 and 2018TC055), the National Key R&D Plan of China (2016YFD0200208) and the NIH (R01GM071779 to O. K.)Notes and references
- (a) D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC; (b) J. P. Michael, Nat. Prod. Rep., 2004, 21, 625 RSC.
- For selected reviews, see: (a) D. O'hagan, Nat. Prod. Rep., 1997, 14, 637 RSC; (b) S. Singh, J. Goo, V. Gajulapati, T.-S. Chang, K. Lee and Y. Choi, Anti-Cancer Agents Med. Chem., 2016, 16, 539 CrossRef CAS.
- For selected examples, see: (a) W. E. Berger, S. Shah, P. Lieberman, J. Hadley, D. Price, U. Munzel and S. Bhatia, Journal of Allergy and Clinical Immunology: In Practice, 2014, 2, 179 CrossRef PubMed; (b) O. O. Daramola and R. C. Kern, Curr. Opin. Otolaryngol. Head Neck Surg., 2016, 24, 10 CrossRef PubMed.
- B. Holmes and A. Ward, Drugs, 1985, 30, 285 CrossRef CAS PubMed.
- For selected reviews, see: (a) M. Gassel, C. Breitenlechner, S. Herrero, R. Engh and D. Bossemeyer, Handb. Exp. Pharmacol., 2005, 167, 85 CAS; (b) I. Collins, Anti-Cancer Agents Med. Chem., 2009, 9, 32 CrossRef CAS.
- For selected recent reviews, see: (a) Z. Li, Q. Guo, L. Wang, S. Wang, X. Zhang, Y. Wang, Z. Gao and C. Jin, Iran. J. Public Health, 2014, 43, 1616 Search PubMed; (b) N. Djordjevic, S. M. Jankovic and J. R. Milovanovic, Eur. J. Drug Metab. Pharmacokinet., 2017, 42, 729 CrossRef CAS PubMed.
- For selected reviews, see: (a) K. Tasaka, Drugs Today, 2000, 36, 735 CrossRef CAS PubMed; (b) F. W. Fraunfelder, Drugs Today, 2004, 40, 677 CrossRef CAS PubMed.
- (a) T. Komoda, Y. Sugiyama, N. Abe, M. Imachi, H. Hirota and A. Hirota, Tetrahedron Lett., 2003, 44, 1659 CrossRef CAS; (b) T. Komoda, K. Yoshida, N. Abe, Y. Sugiyama, M. Imachi, H. Hirota, H. Koshino and A. Hirota, Biosci., Biotechnol., Biochem., 2004, 68, 104 CrossRef CAS PubMed.
- For recent reviews, see: (a) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (b) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (c) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (d) Y. M. Xiao, Z. H. Sun, H. C. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed; (e) Y. Xiao, H. Guo and O. Kwon, Aldrichimica Acta, 2016, 49, 3 CAS; (f) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed; (g) W. Li and J. Zhang, Chem. Soc. Rev., 2016, 45, 1657 RSC; (h) Y. Wei and M. Shi, Org. Chem. Front., 2017, 4, 1876 RSC; (i) H. Chan, W.-L. Ni and Y. Lu, Chem. Rev., 2018, 118, 9344 CrossRef PubMed; (j) H. Guo, Y. C. Fan, Z. Sun, Y. Wu and O. Kwon, Chem. Rev., 2018, 118, 10049 CrossRef CAS PubMed.
- For application in synthesis of natural products, see: (a) J. C. Wang and M. J. Krische, Angew. Chem., Int. Ed., 2003, 42, 5855 CrossRef CAS PubMed; (b) K. Agapiou and M. J. Krische, Org. Lett., 2003, 5, 1737 CrossRef CAS PubMed; (c) Y. S. Tran and O. Kwon, Org. Lett., 2005, 7, 4289 CrossRef CAS PubMed; (d) R. A. Jones and M. J. Krische, Org. Lett., 2009, 11, 1849 CrossRef CAS PubMed; (e) M. Sampath, P.-Y. B. Lee and T. P. Loh, Chem. Sci., 2011, 2, 1988 RSC; (f) I. P. Andrews and O. Kwon, Chem. Sci., 2012, 3, 2510 RSC; (g) R. A. Villa, Q. H. Xu and O. Kwon, Org. Lett., 2012, 14, 4634 CrossRef CAS PubMed; (h) G. A. Barcan, A. Patel, K. N. Houk and O. Kwon, Org. Lett., 2012, 14, 5388 CrossRef CAS PubMed; (i) L. Cai, K. Zhang and O. Kwon, J. Am. Chem. Soc., 2016, 138, 3298 CrossRef CAS PubMed.
- C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS.
- For some reviews, see: (a) W. McCoull and F. A. Davis, Synthesis, 2000, 1347 CrossRef CAS; (b) P. Müller and C. Fruit, Chem. Rev., 2003, 103, 2905 CrossRef PubMed; (c) Y. Tang, S. Ye and X.-L. Sun, Synlett, 2005, 2720 CrossRef CAS; (d) I. D. G. Watson, L. Yu and A. K. Yudin, Acc. Chem. Res., 2006, 39, 194 CrossRef CAS PubMed; (e) G. S. Singh, M. D'hooghe and N. De Kimpe, Chem. Rev., 2007, 107, 2080 CrossRef CAS PubMed; (f) G. Callebaut, T. Meiresonne, N. De Kimpe and S. Mangelinckx, Chem. Rev., 2014, 114, 7954 CrossRef CAS PubMed.
- For selected examples, see: (a) I. Ungureanu, P. Klotz and A. Mann, Angew. Chem., Int. Ed., 2000, 39, 4615 CrossRef CAS; (b) M. Nakagawa and M. Kawahara, Org. Lett., 2000, 2, 953 CrossRef CAS PubMed; (c) R. J. Madhushaw, C.-C. Hu and R.-S. Liu, Org. Lett., 2002, 4, 4151 CrossRef CAS PubMed; (d) V. K. Yadav and V. Sriramurthy, J. Am. Chem. Soc., 2005, 127, 16366 CrossRef CAS PubMed; (e) T. Munegumi, I. Azumaya, T. Kato, H. Masu and S. Saito, Org. Lett., 2006, 8, 379 CrossRef CAS PubMed; (f) P. A. Wender and D. Strand, J. Am. Chem. Soc., 2009, 131, 7528 CrossRef CAS PubMed; (g) B. Kang, A. W. Miller, S. Goyal and S. T. Nguyen, Chem. Commun., 2009, 3928 RSC; (h) J. Fan, L. Gao and Z. Wang, Chem. Commun., 2009, 5021 RSC; (i) F. Fontana, C. C. Chen and V. K. Aggarwal, Org. Lett., 2011, 13, 3454 CrossRef CAS PubMed; (j) R. Maeda, R. Ishibashi, R. Kamaishi, K. Hirotaki, H. Furuno and T. Hanamoto, Org. Lett., 2011, 13, 6240 CrossRef CAS PubMed; (k) G. Arena, C. C. Chen, D. Leonori and V. K. Aggarwal, Org. Lett., 2013, 15, 4250 CrossRef CAS PubMed; (l) R. A. Craig, N. R. O'Connor, A. F. G. Goldberg and B. M. Stoltz, Chem.–Eur. J., 2014, 20, 4806 CrossRef CAS PubMed; (m) M. Yoshiki, R. Ishibashi, Y. Yamada and T. Hanamoto, Org. Lett., 2014, 16, 5509 CrossRef CAS PubMed; (n) J. A. Johnson, B. M. Petersen, A. Kormos, E. Echeverría, Y.-S. Chen and J. Zhang, J. Am. Chem. Soc., 2016, 138, 10293 CrossRef CAS PubMed.
- (a) K. M. Goodenough, P. Raubo and J. P. A. Harrity, Org. Lett., 2005, 7, 2993 CrossRef CAS PubMed; (b) L. C. Pattenden, R. A. J. Wybrow, S. A. Smith and J. P. A. Harrity, Org. Lett., 2006, 8, 3089 CrossRef CAS PubMed; (c) S. Wang, Z. Chai, S. Zhou, S. Wang, X. Zhu and Y. Wei, Org. Lett., 2013, 15, 2628 CrossRef CAS PubMed; (d) S. R. Pathipati, V. Singh, L. Eriksson and N. Selander, Org. Lett., 2015, 17, 4506 CrossRef CAS PubMed.
- R. Shintani, K. Ikehata and T. Hayashi, J. Org. Chem., 2011, 76, 4776 CrossRef CAS PubMed.
- H. Liu, H. Jia, W. Shi, C. Wang, C. Zhang and H. Guo, Org. Lett., 2018, 20, 3570 CrossRef CAS PubMed.
- (a) H. Guo, Q. Xu and O. Kwon, J. Am. Chem. Soc., 2009, 131, 6318 CrossRef CAS PubMed ; for other phosphine-catalyzed [3 + 3] cycloaddition, see: ; (b) S. Zheng and X. Lu, Tetrahedron Lett., 2009, 50, 4532 CrossRef CAS; (c) R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, Y. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard III, H. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337 CrossRef CAS PubMed; (d) J. Liu, H. Liu, R. Na, G. Wang, Z. Li, H. Yu, M. Wang, J. Zhong and H. Guo, Chem. Lett., 2012, 41, 218 CrossRef CAS; (e) J. Hu, W. Dong, X.-Y. Wu and X. Tong, Org. Lett., 2012, 14, 5530 CrossRef CAS PubMed; (f) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316 CrossRef CAS PubMed; (g) Z. Li, H. Yu, Y. Liu, L. Zhou, Z. Sun and H. Guo, Adv. Synth. Catal., 2016, 358, 1880 CrossRef CAS; (h) L. Liang and Y. Huang, Org. Lett., 2016, 18, 2604 CrossRef CAS PubMed; (i) L. Zhou, C. Yuan, C. Zhang, L. Zhang, Z. Gao, C. Wang, H. Liu, Y. Wu and H. Guo, Adv. Synth. Catal., 2017, 359, 2316 CrossRef CAS; (j) W. Yang, W. Sun, C. Zhang, Q. Wang, Z. Guo, B. Mao, J. Liao and H. Guo, ACS Catal., 2017, 7, 3142 CrossRef CAS; (k) L. Zhang, W. Yang, L. Zhou, H. Liu, J. Huang, Y. Xiao and H. Guo, J. Heterocycl. Chem., 2017, 54, 3377 CrossRef CAS.
- For phosphine-catalyzed [4 + 3] annulation, see: (a) K. Kumar, R. Kapoor, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 2023 CrossRef CAS PubMed; (b) S. Zheng and X. Lu, Org. Lett., 2009, 11, 3978 CrossRef CAS PubMed; (c) C. Jing, R. Na, B. Wang, H. Liu, L. Zhang, J. Liu, M. Wang, J. Zhong, O. Kwon and H. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS PubMed; (d) R. Zhou, J. Wang, C. Duan and Z. He, Org. Lett., 2012, 14, 6134 CrossRef CAS PubMed; (e) G. Zhan, M. L. Shi, Q. He, W. Du and Y. C. Chen, Org. Lett., 2015, 17, 4750 CrossRef CAS PubMed; (f) Z. Li, H. Yu, Y. Feng, Z. Hou, L. Zhang, W. Yang, Y. Wu, Y. Xiao and H. Guo, RSC Adv., 2015, 5, 34481 RSC; (g) C. Yuan, L. Zhou, M. Xia, Z. Sun, D. Wang and H. Guo, Org. Lett., 2016, 18, 5644 CrossRef CAS PubMed; (h) J. Chen and Y. Huang, Org. Lett., 2017, 19, 5609 CrossRef CAS PubMed.
- (a) Z. Li, H. Yu, H. Liu, L. Zhang, H. Jiang, B. Wang and H. Guo, Chem.–Eur. J., 2014, 20, 1731 CrossRef CAS PubMed; (b) H. Yu, L. Zhang, Z. Li, H. Liu, B. Wang, Y. Xiao and H. Guo, Tetrahedron, 2014, 70, 340 CrossRef CAS; (c) Z. Gao, C. Wang, C. Yuan, L. Zhou, Y. Xiao and H. Guo, Chem. Commun., 2015, 51, 12653 RSC; (d) C. Wang, Z. Gao, L. Zhou, C. Yuan, Z. Sun, Y. Xiao and H. Guo, Org. Lett., 2016, 18, 3418 CrossRef CAS PubMed; (e) H. Liu, Y. Liu, C. Yuan, G.-P. Wang, S.-F. Zhu, Y. Wu, B. Wang, Z. Sun, Y. Xiao, Q. Zhou and H. Guo, Org. Lett., 2016, 18, 1302 CrossRef CAS PubMed; (f) Y. Liu, W. Yang, Y. Wu, B. Mao, X. Gao, H. Liu, Z. Sun, Y. Xiao and H. Guo, Adv. Synth. Catal., 2016, 358, 2867 CrossRef CAS; (g) Y. Wu, Y. Liu, W. Yang, H. Liu, L. Zhou, Z. Sun and H. Guo, Adv. Synth. Catal., 2016, 358, 3517 CrossRef CAS; (h) B. Mao, W. Shi, J. Liao, H. Liu, C. Zhang and H. Guo, Org. Lett., 2017, 19, 6340 CrossRef CAS PubMed; (i) H. Liu, Y. Zhao, Z. Li, H. Jia, C. Zhang, Y. Xiao and H. Guo, RSC Adv., 2017, 7, 29515 RSC; (j) X. Gao, Z. Li, W. Yang, Y. Liu, W. Chen, C. Zhang, L. Zheng and H. Guo, Org. Biomol. Chem., 2017, 15, 5298 RSC; (k) L. Zhou, C. Yuan, Y. Zeng, H. Liu, C. Wang, X. Gao, Q. Wang, C. Zhang and H. Guo, Chem. Sci., 2018, 9, 1831 RSC; (l) C. Wang, Z. Gao, L. Zhou, Q. Wang, Y. Wu, C. Yuan, J. Liao, Y. Xiao and H. Guo, Chem. Commun., 2018, 54, 279 RSC; (m) Z. Gao, C. Wang, L. Zhou, C. Yuan, Y. Xiao and H. Guo, Org. Lett., 2018, 20, 4302 CrossRef CAS PubMed; (n) L. Zhou, C. Wang, C. Yuan, H. Liu, C. Zhang and H. Guo, Org. Lett., 2018, 20, 6591 CrossRef CAS PubMed.
- Crystallographic data for 4a have been deposited with the Cambridge Crystallographic Data Centre as deposition number CCDC 1869166.†.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data and crystallographic data. CCDC 1869166. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra09852b |
| ‡ Honglei Liu and Yan Lin contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |