Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tetra-fluorinated aromatic azide for highly efficient bioconjugation in living cells†
Xuekang
Cai‡
ab,
Dan
Wang‡
c,
Yasi
Gao
b,
Long
Yi
 bd,
Xing
Yang
bd,
Xing
Yang
 *ae and
Zhen
Xi
*cd
*ae and
Zhen
Xi
*cd
aDepartment of Nuclear Medicine, Peking University First Hospital, Beijing, 100034, China. E-mail: yangxing2017@bjmu.edu.cn
bState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, 15 Beisanhuan East Road, Chaoyang District, Beijing 100029, China
cState Key Laboratory of Elemento-Organic Chemistry, Department of Chemical Biology, National Pesticide Engineering Research Center (Tianjin), Nankai University, Tianjin, 300071, China. E-mail: zhenxi@nankai.edu.cn; Tel: +86 22 23504782
dCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), China
ePeking University School of Medical Technology, Beijing, 100191, China
First published on 19th December 2018
Abstract
We developed a fast strain-promoted azide–alkyne cycloaddition reaction (SPAAC) by tetra-fluorinated aromatic azide with a kinetic constant of 3.60 M−1 s−1, which is among the fastest SPAAC ligations reported so far. We successfully employed the reaction for covalent labelling of proteins with high efficiency and for bioimaging of mitochondria in living cells. The reaction could be a generally useful toolbox for chemical biology and biomaterials.
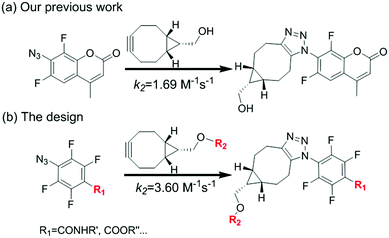
Strain-promoted azide–alkyne cycloaddition (SPAAC) as an efficient bioconjugation tool has recently found a wide range of applications in chemical biology, materials science and medical science.1–3 In 2001, Sharpless et al., first introduced the concept of click chemistry that emphasized on the rapid synthesis of useful new compounds and combinatorial libraries through heteroatom links (C–X–C).4 To overcome the limitations for classical copper-catalyzed azide–alkyne cycloaddition (CuAAC),5 Bertozzi improved the method using strained cyclooctynes to react with azide in 2004, which effectively avoids the transition metal catalysts (such as Cu) and makes it more general for bioconjugations.6,7 It gave birth to the development of SPAAC. But one of the limitations is the relative low reaction rate compared with other biorthogonal reactions and a high rate could be beneficial for labelling low abundance or transient structures with short-lived signal molecules, such as radioisotopes for imaging.8 During the past decade, efforts have been made to further improve the method. Structure modified cyclooctynes, including aliphatic cyclooctynes9 and (di)benzoannulated cyclooctynes10 have been investigated to accelerate the reaction, and recently the modified azides was also reported to remarkably increase SPAAC rate.11
We have been interested in the development of fast chemoselective reactions for bioconjugations.12 We reported o,o′-difluorinated aromatic azide can accelerate both the reaction rate significantly on SPAAC and H2S-mediated reduction of the azide.12c Therefore, we envision that multi-fluorinated aromatic azide may have interesting properties for fast and convenient bifunctional conjugation. 4-Azido-2,3,5,6-tetrafluorobenzoic acid (1) was selected for investigation. Here, we report the SPAAC kinetic properties of 1 and its applications for protein labelling in vitro and for bioimaging of subcellular organelles in living cells (Fig. 1).
To test our idea, the reaction between 1 and [1R,8R,9S]-bicyclo[6.1.0]non-4-yn-9-yl methanol (2) was studied for SPAAC reaction, monitored with time-dependent 1H NMR. To our delight, the reaction could finish within 5 min. As shown in Fig. 2, the 1H-NMR signal of 2 (40 mM) completely changed to the product (3) after adding 1 for 5 min, and there's no further change at 15 min and 2 hours in NMR spectra. The resulted product was also confirmed by high resolution mass spectrum. This result implies that the 4-azido-2,3,5,6-tetrafluorobenzoic acid derivatives can be used for this fast SPAAC reaction. But NMR is limited by its relative low sensitivity for accurate testing the kinetic rate at micromolar concentration.
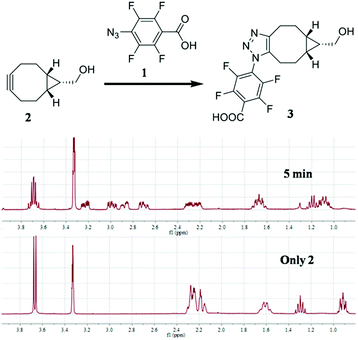 | ||
| Fig. 2 1H NMR analysis of the reaction between 1 (120 mM) and 2 (40 mM). The reaction was carried out in CD3OD. | ||
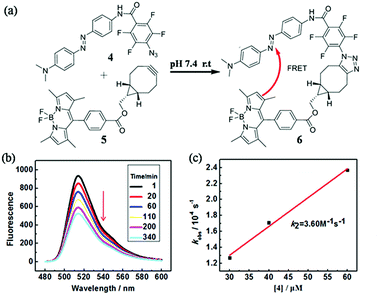
In order to quantify the kinetic rate, we designed a procedure based on fluorescence resonance energy transfer (FRET) method to monitor this fast SPAAC reaction. We synthesized the azide compound 4 conjugated to an azo-quencher and the cyclooctyne compound 5 conjugated to a BODIPY-dye (Fig. 3). All the compounds were isolated and characterized by NMR and HRMS. After the reaction between 4 and 5, 6 could formed in which BODIPY fluorescent signal was quenched due to the FRET effect. Such fluorescence change could be employed to monitor the reaction in a real-time.
The reaction between 4 and 5 was set up in PBS buffer (2 μM 5, 50 mM, pH 7.4) and the maximum emission at 511 nm was monitored with the excitation at 473 nm. The pseudo-first-order rate kobs was determined by fitting the data with a single exponential function. The linear fitting between kobs and the concentrations of 4 gave the reaction rate (k2) as 3.60 M−1 s−1. It's two-fold faster than o,o′-difluorinated aromatic azide that we reported earlier, 1500-fold faster than the original SPAAC reaction and among the fastest SPAAC reactions reported so far.
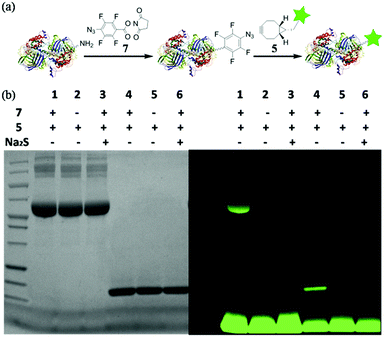
With this highly efficient reaction, covalent protein labelling was first tested as its application. N-Hydroxysuccinimide ester of 4-azido-2,3,5,6-tetrafluorobenzoic acid was synthesized as bifunctional labelling compound (7) for amide and SPAAC reactions. We chose bovine serum albumin (BSA) and lysozyme as model proteins considering their different sizes and functions.8 As shown in Fig. 4a, we tested to label protein first with 7 and then conjugated a dye to the protein using fluorescent cyclooctyne (5). BSA or lysozyme was treated with 0.5 mM 7 in PBS buffer (50 mM, pH 8.5, containing 10% DMSO) for 2 h to incorporate tetra-fluorinated aromatic azide into the protein. After removing of small molecules, the azide labelled protein was incubated with 1 mM 5 for another 2 h to achieve SPAAC protein fluorescent dye labelling. As a control, the azide labelled protein was also treated with Na2S for 10 minutes to reduce azide into amine before incubating with 5, so to prove the reaction specificity. The labelled proteins were analysed with SDS-PAGE either stained by Coomassie blue or excited under UV lamp to visualize the desired protein. The results are shown in Fig. 4b. The strong fluorescent labelled BSA (lane 1) and lysozyme (lane 4) could be observed after the reaction with tandem addition of 7 and 5, while the controls of 5 only (lane 2 and 5) and Na2S treated labelling (lane 3 and 6) did not show any fluorescent signal. The results indicated that tetra-fluorinated aromatic azide-cyclooctyne is a highly efficient SPAAC reaction for protein labelling.
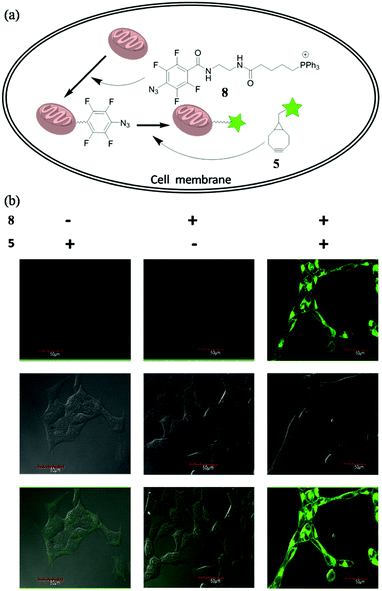
To future explore the biocompatibility of this SPAAC reaction in living cells, we tested it for bioimaging of the subcellular organelle mitochondria. Triphenylphosphines are known to enrich into mitochondria mainly due to its positive charged character.13 We designed and synthesized compound 8, with the azide conjugated to a triphenylphosphine. Upon incubating 8 with the cells, we expected it would target mitochondria, so we can use SPAAC reaction to label the cells with fluorescent compound 5 (Fig. 5a). Human embryonic kidney cells 293 (HEK 293) were chosen for the experiment. The cells were first treated with 8 (10 μM) for 20 min to accumulate tetra-fluorinated aromatic azide into mitochondria, and then incubated with 5 μM 5 for another 30 min. After the non-specific fluorescent signal was washed away, the cells were imaged under the excitation of 488 nm. The experimental conditions and results were shown in Fig. 5b. The cells could only be fluorescence-labelled when treated with both 8 and 5, and the imaging signal all localized on mitochondria. The result proved both the feasibility and biocompatibility for the application of this improved SPAAC conjugation at cellular level. Its superior kinetic character (k2 of 3.60 M−1 s−1) may even enable in vivo applications for pretargeted imaging,14 which is currently under investigation.
Conclusions
In summary, we investigated tetra-fluorinated aromatic azide for SPAAC reactions with cyclooctyne. The reaction rate could reach 3.60 M−1 s−1 for 4 and 5, which is among the fastest SPAAC reactions reported so far. The commercially available agent 4-azido-2,3,5,6-tetrafluorobenzoic acid (1) enables its convenient application as bifunctional biorthogonal tool. We have successfully employed the reaction for covalent labelling of proteins and the mitochondria in living cell. In vivo applications are currently under investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the MOST (2017YFD0200900), NSFC (21572019, 21877008), 111 project (B14004), National Thousand Young Talents Program.Notes and references
- (a) C. P. R. Hackenberger and D. Schwarze, Angew. Chem., Int. Ed., 2008, 47, 10030 CrossRef CAS; (b) D. Schumacher, J. Helma, A. F. L. Schneider, H. Leonhardt and C. P. R. Hackenberger, Angew. Chem., Int. Ed., 2018, 57, 2314 CrossRef CAS; (c) C. Witte, V. Martos, H. M. Rose, S. Reinke, S. Klippel, L. Schrçder and C. P. R. Hackenberger, Angew. Chem., Int. Ed., 2015, 54, 2806 CrossRef CAS.
- (a) S. V. Orski, A. A. Poloukhtine, S. Arumugam, L. D. Mao, V. V. Popik and J. Locklin, J. Am. Chem. Soc., 2010, 132, 11024 CrossRef CAS; (b) W. Luo, P. Gobbo, C. D. McNitt, D. A. Sutton, V. V. Popik and M. S. Workentin, Chem.–Eur. J., 2017, 23, 1052 CrossRef CAS; (c) C. A. DeForest and K. S. Anseth, Nat. Chem., 2011, 3, 925 CrossRef CAS PubMed.
- (a) A. Wurzer, C. Seidl, A. Morgenstern, F. Bruchertseifer, M. Schwaiger, H.-J. Wester and J. Notni, Chem.–Eur. J., 2018, 24, 547 CrossRef CAS PubMed; (b) Z. Zhou, S. K. Chitneni, N. Devoogdt, M. R. Zalutsky and G. Vaidyanathan, Bioorg. Med. Chem., 2018, 26, 1939 CrossRef CAS PubMed; (c) A. Wurzer, J. Pollmann, A. Schmidt, D. Reich, H.-J. Wester and J. Notni, Mol. Pharm., 2018, 15, 4296 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046 CrossRef CAS PubMed.
- G. Wittig and A. Krebs, Chem. Ber., 1961, 94, 3260 CrossRef CAS.
- G. S. McKay and M. G. Finn, Chem. Biol., 2014, 21, 1075 CrossRef PubMed.
- (a) J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422 CrossRef CAS; (b) J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793 CrossRef CAS; (c) N. J. Agard, J. M. Baskin, J. A. Prescher, A. Lo and C. R. Bertozzi, ACS Chem. Biol., 2006, 1, 644 CrossRef CAS.
- (a) X. Ning, J. Guo, M. A. Wolfert and G.-J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253 CrossRef CAS; (b) J. C. Jewett, E. M. Sletten and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 3688 CrossRef CAS PubMed; (c) M. F. Debets, S. S. van Berkel, S. Schoffelen, F. P. J. T. Rutjes, J. C. M. van Hest and F. L. van Delft, Chem. Commun., 2010, 46, 97 RSC; (d) A. Kuzmin, A. Poloukhtine, M. A. Wolfert and V. V. Popik, Bioconjugate Chem., 2010, 21, 2076 CrossRef CAS PubMed.
- J. Dommerholt, O. van Rooijen, A. Borrmann, C. F. Guerra, F. M. Bickelhaupt and F. L. van Delft, Nat. Commun., 2014, 5, 5378 CrossRef CAS PubMed.
- (a) Y. Xie, L. Cheng, Y. Gao, X. Cai, X. Yang, L. Yi and Z. Xi, Chem.–Asian J., 2018, 13, 1791 CrossRef CAS PubMed; (b) D. Ma, Y. Xie, J. Zhang, D. Ouyang, L. Yi and Z. Xi, Chem. Commun., 2014, 50, 15581 RSC; (c) J. Zhang, Y. Gao, X. Kang, Z. Zhu, Z. Wang, Z. Xi and L. Yi, Org. Biomol. Chem., 2017, 15, 4212 RSC.
- N. K. Roopa, V. Bhalla and M. Kumar, Chem. Commun., 2015, 51, 15614 RSC.
- J.-P. Meyer, P. Adumeau, J. S. Lewis and B. M. Zeglis, Bioconjugate Chem., 2016, 27, 2791 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional figures. See DOI: 10.1039/c8ra09303b |
| ‡ The authors pay equal contributions to this work. |
| This journal is © The Royal Society of Chemistry 2019 |