Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silver ferrite: a superior oxidizer for thermite-driven biocidal nanoenergetic materials†
Tao Wu and
Michael R. Zachariah
and
Michael R. Zachariah *
*
Department of Chemistry and Biochemistry, Department of Chemical and Biomolecular Engineering, University of Maryland, College Park, MD 20742, USA. E-mail: mrz@umd.edu
First published on 14th January 2019
Abstract
Silver-containing oxidizers are of interest as biocidal components in energetic application such as thermites due to their biocidal agent delivery. In this study, AgFeO2, was evaluated as an oxidizer in aluminum-based thermite system. This novel oxidizer AgFeO2 particles were prepared via a wet-chemistry method and its structure, morphologies and thermal behavior were investigated using X-ray diffraction, scanning electron microscopy, thermogravimetric analysis and differential scanning calorimetry, and time-resolved temperature-jump time-of-flight mass spectrometry. The results indicate the decomposition pathways of AgFeO2 vary with heating rates from a two-step at low heating rate to a single step at high heating rate. Ignition of Al/AgFeO2 occurs at a temperature just above the oxygen release temperature that is very similar to Al/Fe2O3 and Al/CuO. However, with a pressurization rate three times of Al/CuO, Al/AgFeO2 yields a comparable result as to Al/hollow-CuO or Al/KClO4/CuO, with a simpler preparation method. The post combustion products demonstrated that the Al/AgFeO2 thermite reaction produces a fine dispersion of elemental nanosized silver particles which coats the larger alumina particles and is thus bioavailable.
Introduction
High efficiency neutralization of biological warfare agents has become of increased importance due to the enhanced threat of bioterrorism.1–4 Preliminary laboratory studies have suggested that an ideal neutralization process should contain not only a high thermal event but also a long-lasting biocidal agent release.5–11 The main problem with conventional energetic materials is low neutralization efficiency since a thermal neutralization mechanism is nominally dominant.5 Therefore, it has been proposed that simultaneously delivering a rapid thermal pulse with a remnant biocidal agent would prolong the exposure time and improve the inactivation process.12Reactive materials are a class of energetic materials containing separated fuels and oxidizers and featuring an extremely high exothermicity, intensive light emission and shock generation by the self-sustained reaction.13–15 When metal and metal oxide are employed as the fuel and oxidizer, respectively, the intense exothermic metal/metal oxide reaction is commonly referred to a thermite reaction. This reaction is self-propagating once the ignition starts and the kinetics of the reaction are known to be accelerated when the fuel and oxidizer are at the nanoscale, resulting from the increased interfacial contact and reduced characteristic diffusion length scale. The most widely adopted metal is aluminum due to its abundance and competitive reactivity when compared with other metals.16 Various oxidizers, such as metal oxides,17–26 iodine oxides,27–32 sulfates,33,34 iodates,12,35,36 potassium salts,37,38 and etc., have been employed in formulation of thermites. Among all, halogens-containing and silver-containing oxidizers have drawn attention because of their excellent biocidal properties.39–43 For halogens, iodine stands out owing to its strong neutralization effect and different iodine-containing oxy-compounds, such as metal iodates and iodine oxides,5,12,18–25,27–33,35–37,44 have been studied in thermite systems.
As to silver, it has been pointed out that silver exhibits biocidal properties in the forms of metallic Ag particles and silver ions in a humid environment.45 Since most of the products of thermite combustion tend to be primarily in the condensed phase,2 metallic silver particles are the focus of this work. As indicated by Morones et al.46 and Smetana et al.,47 small particle sizes are necessary for silver particles to perform well in biocidal activities. To deliver not only a high thermal event but also a large amount of small silver particles as the active biocidal sites is the goal of this work.
When it comes to silver-containing oxidizers, Ag2O was the obvious choice to be considered as an oxidizer in an aluminum-based thermite system. In 2010, Clark et al.48 investigated the combustion performance and biocidal abilities of both Al/I2O5 and Al/Ag2O thermites using a homemade biocidal reaction chamber. They concluded Al/I2O5 thermite exhibited significant spore neutralization owing to the generation of lot of iodine gas. In 2011, Russell et al.49 further studied the flame propagation behaviors of Al/I2O5 and Al/Ag2O thermites using mechanical impact and thermal initiation. The results show that Al/Ag2O features a lower average flame propagation by about 2.5 times in thermal ignition tests but produce much more energy than Al/I2O5 in impact-driven ignition tests. They also argued that the energy release of the thermite reactions is significantly enhanced by reducing the sizes of the oxidizers particles. In the same year, Sullivan et al.5 investigated the performance of AgIO3 as an oxidizer in aluminum-based thermite because it decomposed to O2, O and I gases when heated at an ultra-high heating rate. Silver was not observed in the mass spectra probably because the temperature was not high enough to reach the adiabatic flame temperature and thus could not vaporize silver. However, Al/AgIO3 considerably outperformed Al/CuO in pressurization rate due to a large number of gaseous products released from AgIO3; however, its high combustion performance was mitigated by the fact that the reaction products were found to form AgI instead of elemental silver and iodine, thus obstructing its usage in biocidal applications.
Sullivan et al.2 subsequently synthesized nano-Ag2O particles and investigated its reactivity as an oxidizer in biocidal energetic systems since it produces high yields of antimicrobial silver as one of the combustion products. They found that Ag2O alone performs poorly in terms of pressurization rate and burn time, but its performance is significantly improved when combined with one more reactive oxidizer, such as AgIO3 or CuO. The morphology of the final products was also studied and indicated that abundant active sites of silver particles were sacrificed since some silver particles were trapped within the interior of other products, which might to some extent affect its biocidal activity. Inspired by the fact that CuO addition improves the performance of Al/Ag2O significantly, we embed the extra oxidizer into Ag2O molecularly in this work. Since we have some experience on ferrite-type oxides (AFeO4) and delafossite-type oxides (ABO2) previously, AgFeO2 as a molecularly mixed oxidizer of Ag2O and Fe2O3 with a delafossite structure was a good choice. In addition, Fe2O3 has been proven to be a poor oxidizer in Al-based thermite system,37 it could be of significant interest if AgFeO2 which comprises two poor oxides (Ag2O and Fe2O3) became a strong oxidizer.
A wet-chemistry method was adopted here for the preparation of AgFeO2 and its thermal behavior were investigated using a low heating rate thermogravimetric analysis and differential scanning calorimetry in an argon environment. Time-resolved temperature-jump time-of-flight mass spectrometry (T-Jump/TOFMS) was also employed to evaluate the decomposition behaviors of bare AgFeO2 or Al/AgFeO2 thermites under rapid heating rates, enabling us to probe the reaction process on a time scale close to that of a combustion event. The results indicate that the decomposition pathways of AgFeO2 vary in term of heating rates. For a comparison purpose, Al/CuO, Al/Ag2O and Al/Ag2O/Fe2O3 were also included in this work. A high-speed camera coupled with T-Jump/TOFMS simultaneously captured optical emission from the ignition/reaction of the thermites allowing us to obtain the ignition time, and corresponding ignition temperature. In addition, constant volume combustion cell tests were performed on aluminum-based thermites. The post combustion products were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and energy-disperse X-ray spectroscopy. The results demonstrated that the Al/AgFeO2 thermite reaction produce an enormous amount of nanosized silver particles and feature the best combustion performance in this work.
Materials and characterizations
Materials
The aluminum nanopowders (Al) (Alex, ∼80 nm) was purchased from Novacentrix. The active Al was 81% by mass, determined by TGA. All metal oxide nanopowders (<50 nm) purchased from Sigma-Aldrich was directly used as received. All the other chemicals were of analytical grade and used as purchased without further treatment.Preparation of AgFeO2
AgFeO2 powders were prepared via a co-precipitation method. For this, 2.66 mmol of Fe(NO3)3·9H2O and 2.66 mmol of AgNO3 were dissolved in 20 mL of water and stirred for 30 minutes. The solution was then heated to 80 °C and stirred for one hour. Then, 1.5 M of NaOH solution was added dropwise into the solution until its pH reaches 13, followed by another 6 hours of stirring on a hot plate (80 °C). The prepared AgFeO2 powders could be easily isolated from the solution by vacuum filtration and were purified by successively washing with copious amount of distilled water and absolute ethanol. Finally, the product was dried in an oven at 70 °C.Preparation of Ag2O
Ag2O powders were prepared by adding 0.025 M NaOH solution dropwise into 80 mL of AgNO3 solution (0.005 M) with stirring until the solution become a grey–yellow colloidal suspension. The suspension was kept at 60 °C for another 2 hours to ensure complete reaction. The prepared Ag2O powders were collected by centrifugation and washed with distilled water and then absolute ethanol three times. The solid pure Ag2O was obtained after being dried in an oven at 70 °C for 10 hours.Preparation of thermites
Aluminum nanopowders was stoichiometric mixed with AgFeO2, Ag2O, CuO and Fe2O3 based on the following equations, respectively, in dry hexane followed by 30 minutes sonication (Table 1). After room temperature evaporation of the solvent the solid thermite powders were collected.| Thermite reaction | Heat of reaction (cal g−1) |
|---|---|
| a The thermodynamic data of AgFeO2 is unknown. Data is taken from Fischer and Grubelich50 without taking account of the oxide shell on aluminum. | |
| 2Al + 3Ag2O → 6Ag (l, g) + Al2O3 (l) | 504.8 |
| 2Al + 3CuO → 3Cu (l, g) + Al2O3 (l) | 974.1 |
| 2Al + Fe2O3 → 2Fe (l, g) + Al2O3 (l) | 945.4 |
| 4Al + 3AgFeO2 → 3Ag + 3Fe + 2Al2O3 (l) | |
T-Jump/TOFMS measurement and high-speed imaging
The decomposition of oxides particles was investigated using a custom T-Jump/TOFMS5 Typically, a ∼1 cm long platinum wire (76 μm in width) with a thin coating of oxidizer or thermite sample was rapidly joule-heated to about 1200 °C by a 3 ms pulse at a heating rate of ∼105 °C s−1. The current and voltage signals were recorded, and the temporal temperature on the wire was measured according to the Callendar–Van Dusen equation. MS spectra were measured every 0.1 ms. The detailed experimental set-up is given in our previous papers.5,33To identify the point of ignition a high-speed camera (Vision Research Phantom v12.0) was employed to record the combustion on the wire during heating. Ignition temperatures of thermite reactions in vacuum were measured from the correlation of optical emission from high speed imaging and temporal temperature of the wire and were further analyzed in combination with the temporal mass spectra. Each experiment was repeated 3 times.
X-ray diffraction (XRD) measurement and Rietveld refinement
The as-prepared samples were characterized by powder X-ray diffraction. Diffraction pattern was measured using Cu Kα radiation in Bragg–Brentano geometry on Bruker D8 Advance powder diffractometer equipped with incident beam Soller slits, Ni β-filter and LynxEye position sensitive detector. Data were collected from 10° to 90° 2θ with a step size of 0.01578° and counting time of 1 s per step (total exposure time of 180 s per step).Thermogravimetric analysis/differential scanning calorimetry (TGA/DSC) measurement
Thermogravimetric analysis and differential scanning calorimetry (TGA/DSC) was performed using a TA Instruments SDT Q600. The analysis was performed under a 100 mL min−1 argon flow with ∼1.0 mg samples placed into an alumina pan and heated from room temperature up to 1000 °C at a rate of 10 °C min−1 in argon atmosphere.Morphologies and structures characterizations transmission electronic microscopy (TEM)
Transmission electronic microscopy (TEM, JEOL JEM 2100 FEG) and scanning electronic microscopy (SEM, Hitachi Su-70) were used to investigate morphologies and structures of thermites. Elemental distribution in the thermites was analyzed by Energy-disperse X-rat spectroscopy (EDS) on both SEM and TEM.Combustion test
Combustion properties of themites were evaluated in a constant-volume combustion cell, with simultaneous pressure and optical emission measurements. In this study, 25 mg of thermite powders was loaded inside the cell (constant volume, ∼13 cm3) and ignited by a resistively heated nichrome wire. The temporal pressure and optical emission from the thermite reaction were measured using a piezoelectric pressure sensor and a photodetector, respectively. More detailed information on the combustion cell test can be found in our previous publications.21,25,30 Each experiment was repeated at least 3 times.Results and discussions
Synthesis and characterization of AgFeO2
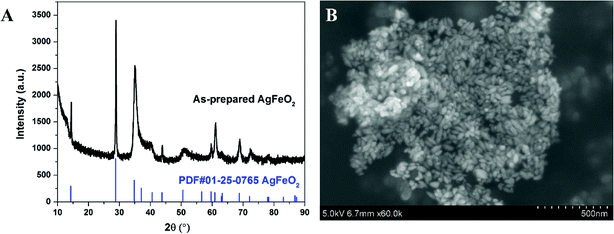
XRD of as-prepared materials shown in Fig. 1A indicates that silver ferrite was successfully prepared. The particles have an oval shape with particle size of ∼40 nm based on the SEM image shown in Fig. 1B, and suggests it might be a very good oxidizer in a thermite system due to its small size.33The thermal stability of AgFeO2 was studied with a TGA in Ar at a heating rate of 10 °C min−1. The result shown in Fig. 2 indicates a ∼4% weigh loss at around 650 °C, corresponding to O2 gas release based on eqn (1). To determine the composition of the remaining material, XRD shown in Fig. 2B indicates the formation of both Fe2O3 and Ag.
| 2AgFeO2 → 2Ag + Fe2O3 + 1/2O2 | (1) |
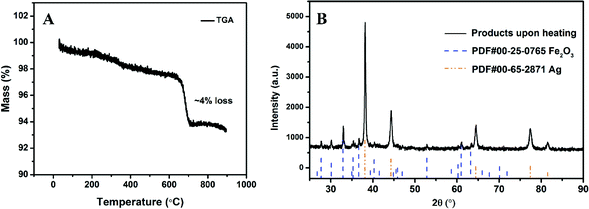 | ||
| Fig. 2 (A) TGA of AgFeO2 in Ar at a heating rate of 10 °C min−1; (B) AgFeO2 decomposition product after heating to 1000 °C. | ||
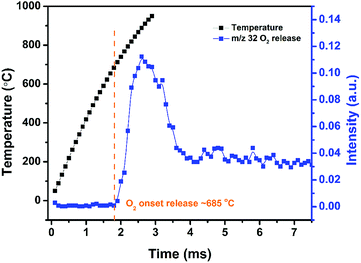
High heating rate decomposition of AgFeO2 was investigated using TOFMS/T-Jump (3 ms, heating rate ∼ 5 × 105 °C s−1). Fig. 3 shows the temporal evolution of O2 from AgFeO2 during rapid heating. Oxygen was first detected at around 685 °C which is slightly higher than the onset decomposition temperature under low heating rate TGA. Only one O2 signal stage is observed indicating the decomposition of AgFeO2 is a one-step event at high heating rates conditions, as compared to its multistage decomposition behavior at low heating rates as discussed previously.
Time-resolved T-Jump mass spectra from rapid heating of AgFeO2 at 1.6–2.2 ms is shown in Fig. S1† to further explore its decomposition process. Apparently, the onset decomposition of AgFeO2 started at around 1.8 ms (685 °C) with the appearance of a small O2 peak. For mass spectra taken at prior times, H2O+, OH+, CO+/N2+ peaks are attributed to the background.18 Above the decomposition temperature, no new peak except for O2 peak is found again suggests a one-step decomposition of AgFeO2 at high heating rate. CO2+ was also observed which we attribute to decomposition products of the precursor salt residue on the AgFeO2 surface.17
Ignition of Al/AgFeO2 nanothermite
To evaluate the performance of AgFeO2 as an oxidizer in Al-based thermite, physical mixtures of nanosized aluminum and AgFeO2 were made, following 30 min sonication of the mixture in dry hexane. Sequential snapshot of Al/AgFeO2 ignition during high-rate heating under vacuum were captured using a high-speed camera and is shown in Fig. 4. Optical emission from Al/AgFeO2 reaction was first observed at 1.662 ms with a corresponding wire temperature of ∼740 °C as a sign of ignition. Multiple ignition points are observed in the prior time and then merge into a large bright flame. One should also notice that, upon ignition, thousands of bright dots were rapidly ejected out from the reactants coated on the Pt wire (even more bright dots appeared when ignited in an argon environment as shown in Fig. S2†). The burn time of the thermite in T-Jump chamber could be roughly obtained based on the visual flame and is about 0.3 ms, which is almost the same as the value obtained from the combustion cell test discussed below.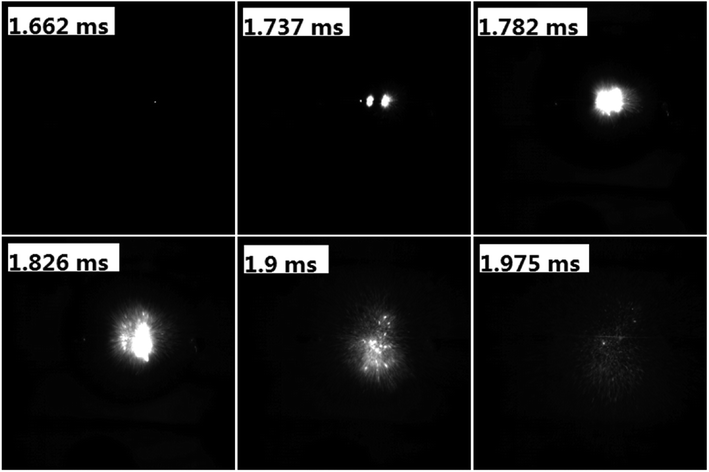 | ||
| Fig. 4 Sequential snapshots of Al/AgFeO2 burning on a high-rate heating Pt wire in T-Jump chamber (in vacuum). The labels in each image are the time elapse after triggering. | ||
Ag2O was included as an oxidizer in Al-based thermites as a control. As the most common used oxidizer, CuO was also included as a standard reference. Since AgFeO2 (Ag2Fe2O4) can be molecularly written as Ag2O and Fe2O3, a binary mixture Ag2O–Fe2O3 with 1/1 molar ratio is also included for comparison.
Fig. 5 shows the relationship between O2 release temperature in neat oxides and the ignition temperature of corresponding thermites under vacuum. There is a good correlation between the oxygen release from the bare oxidizer and ignition of Al/CuO, Al/Fe2O3 and Al/AgFeO2. Al/Fe2O3 has a very high ignition temperature due to the poor performance of Fe2O3 as an oxidizer.24 On the other hand, Al/Ag2O thermite ignited at around ∼660 °C, essentially the melting temperature of aluminum. Considering Ag2O releases oxygen at around 520 °C, it is reasonable to conclude that the ignition of Al/Ag2O thermite is limited to the melting phase of aluminum like most Al/metal oxides systems.19 In fact, the ignition temperatures of the other four samples are all higher than the melting point of aluminum indicates that gaseous oxygen released from the decomposition of oxidizers, was insufficient to ignite aluminum when it is still in the solid phase.
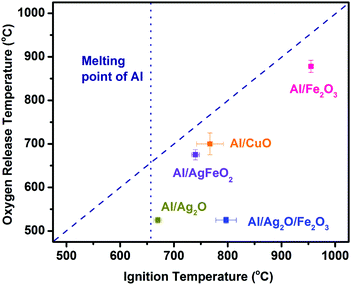 | ||
| Fig. 5 Ignition temperature vs. oxygen release temperature from the corresponding oxidizer. The diagonal dash line presents a perfect correlation. | ||
Al/Ag2O/Fe2O3 ternary thermite ignited at around 800 °C much higher than its oxygen release temperature (520 °C). Considering Al/Ag2O and Al/Fe2O3 ignited at around 660 and 940 °C, respectively, an ignition temperature of about 800 °C is reasonable for the ternary system Al/Ag2O/Fe2O3. The Fe2O3 addition is more likely hindering/weakening the ignition/reaction of the ternary system due to its weak reactivity in Al-based thermites.37
As to Al/AgFeO2, it ignited at a temperature close to the oxygen release temperature of the corresponding oxidizer and higher than the melting temperature of aluminum following an ignition mechanism similar to Al/Fe2O3 and Al/CuO.18,19 Moreover, the fact that the ignition temperature of Al/Fe2O3 is much higher than that of Al/AgFeO2 implies the oxygen involved with Al/AgFeO2 ignition come from AgFeO2 rather than the first-stage decomposed product Fe2O3 as indicated in TGA. Since only one oxygen release stage appeared at the mass spectra and the violent reaction of Al/AgFeO2 at 1.7 ms (∼780 °C) implies decomposition of AgFeO2 is a one-step process during Al/AgFeO2 thermite reaction at high heating rate.
Combustion performance of Al/AgFeO2 nanothermite
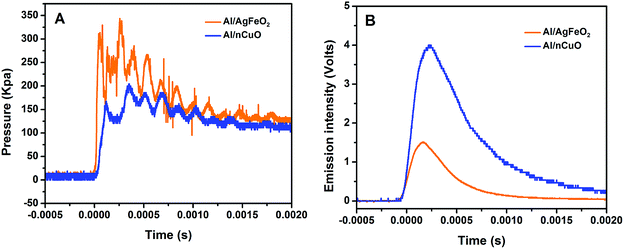
The combustion performance of Al/AgFeO2 at room atmosphere was studied using a constant volume combustion cell, and the results are summarized in Table 2. The pressurization rate and peak pressure in Al/AgFeO2 reaction are 6200 kPa ms−1 and 325 kPa, respectively, which are considerably higher than those of nano-thermite reaction of Al/CuO. A direct comparison between the temporal pressure traces of Al/AgFeO2 and Al/CuO was shown in Fig. 6A in which the first peak was used to determine the corresponding pressurization rate. Clearly, Al/AgFeO2 reaction reaches its first peak with a faster rate and higher peak value compared with Al/CuO. We have claimed in our previous works that gaseous species release from the decomposition of the oxidizer is the main cause for the pressurization, which can occur much earlier than ignition/combustion.37,51 The fact that AgFeO2 (∼16%) has a lower oxygen weight ratio than CuO (∼20%) suggests the O2 release rate of AgFeO2 must be higher than that of CuO in order to output such high pressure. In addition, the optical signals peak much later than the corresponding pressure peaks for both Al/CuO (∼0.5 ms) and Al/AgFeO2 (0.9 ms) implying a similar mechanism.51,52 Therefore, similar to Al/CuO,37 the ignition/reaction mechanism of Al/AgFeO2 is summarized as follows: AgFeO2 releases O2 gas (∼690 °C) and the aluminum core becomes mobile (633 °C); and the mixture ignites (∼740 °C, Fig. 5) and the generated heat further promote the decomposition of AgFeO2 to pressurize the system.| Thermites (stoichiometric) | Peak pressure (kPa) | Pressurization rate (kPa ms−1) | Burn time (ms) | Peak optical emission (volts) |
|---|---|---|---|---|
| Al/nCuO | 226 | 2045 | 0.46 | 4.3 |
| Al/Ag2O | 75 | 25.7 | 3.6 | 0.3 |
| Al/Ag2O/Fe2O3 | 74 | 21 | 5 | 0.4 |
| Al/AgFeO2 | 325 | 6200 | 0.3 | 1 |
Moreover, with a pressurization rate three times of Al/CuO, Al/AgFeO2 yields a comparable result as prior work on Al/hollow-CuO25 or Al/KClO4/CuO,37 which make AgFeO2 a powerful replacement owing to its simpler and cheaper preparation method.
Sullivan et al.2 has previously reported that Al/Ag2O suffers a poor combustion performance in terms of both pressurization rate and peak pressure and incorporation of a small fraction of CuO into the Al/Ag2O system improved its combustion performances extensively to even close to the reactivity of Al/CuO. An explanation was provided by Sullivan et al. that CuO addition can increase the reaction temperature and thus further enhance the performance of Ag2O as an oxidizer. Consistent with this reported result, Al/Ag2O performs poorly in the combustion cell test due to an early release of O2;2 however, Fe2O3 addition does not show much improvement to Al/Ag2O system as CuO does. Instead of improving, Fe2O3 even weaken the combustion performance of Al/Ag2O system considering Al/Ag2O/Fe2O3 features the worst pressurization rate and burn time. Thermodynamic equilibrium predictions of Al/CuO, Al/Ag2O and Al/Fe2O3 thermites by Fischer and Grubelich2,27,50 are shown in Table 3 where we can see that both CuO and Ag2O should perform significantly better than Fe2O3 in terms of gas production. And this is consistent with our experimental data shown in Table 2. In detail, unlike CuO that can rapidly release O2, Fe2O3 cannot efficiently decompose and upon decomposition most of the oxygen is still fixed as solid FeO,37 which leads to its decomposition as the rate-limiting step for Al/Ag2O/Fe2O3 reaction and therefore weakened combustion performance. In fact, Fe2O3 has been introduced previously as a moderator to weaken the reactivities of Al/KMnO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and Al/KClO4.37
24 and Al/KClO4.37
| Thermite reaction | Adiabatic temperature (°C) | Gas production (mmol g−1) | Major gas species |
|---|---|---|---|
| a Assumptions: constant enthalpy and pressure with phase changes; without taking account of the oxide shell of aluminum. Data is taken from Fisher and Grubelich.50 | |||
| 2Al + 3CuO → Al2O3 (l) + 3Cu (l, g) | 2570 | 5.4 | Cu |
| 2Al + 3Ag2O → Al2O3 (l) + 6Ag (l, g) | 2163 | 4.3 | Ag |
| 2Al + Fe2O3 → Al2O3 (l) + 2Fe (l, g) | 2862 | 1.4 | Fe |
Al/AgFeO2 significantly outperforms Al/Ag2O/Fe2O3 by a scale of almost 100 in pressurization rate and about 4 in peak pressure. The fact that Al/AgFeO2 and Al/Ag2O/Fe2O3 share the exact same elemental compositions indicates molecularly incorporation of Ag2O into Fe2O3 outperforms the mechanically mixed Ag2O/Fe2O3 when they were employed as oxidizers in aluminum-based thermites.
The burn time of the thermite reactions measured in the combustion cell are shown in Table 2. Clearly, Al/AgFeO2 features the shortest burn time which makes it the most violent thermite among the four examined. A direct comparison of the optical emission trace of Al/AgFeO2 and Al/CuO was shown in Fig. 6B. The Al/CuO reaction has a four-times higher peak optical emission but 0.1 ms longer burn time compare with those of Al/AgFeO2. This result indicates that Al/AgFeO2 is a weaker heat generator than Al/CuO; but reacts more rapidly. In general, AgFeO2 is the best oxidizer among those four in the aluminum-based thermite system from both pressurization and optical emission perspectives.
Post-combustion-product characterization
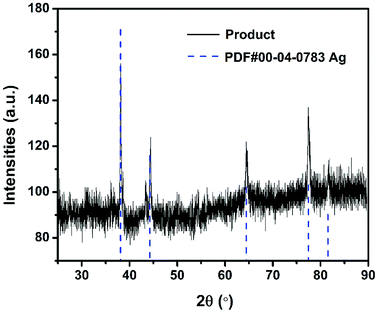
It has been pointed out previously that the nature and dispersion of the products from a thermite combustion plays an important role in biocidal applications.27 XRD evaluation of crystalline product species is shown in Fig. 7 and show no evidence of the parent starting materials, but five new strong peaks indexed to elemental silver are seen. The fact that no peak corresponding to Al2O3 or Fe was observed might suggest they are both amorphous.For SEM evaluation of the reaction product a 7 mm × 5 mm rectangular double-sided carbon tape was placed inside the combustion cell chamber. Two representative SEM images of the product were shown in Fig. 8A and B, there are mainly two populations of product particle sizes. One has relatively larger dimension and another with a dimension as small as ∼80 nm. 2D elemental mapping using EDS shown in Fig. 8, indicate the larger spherical particles are Al2O3 while the smaller particles are Ag. This was also confirmed with 1D elemental line-scan coupled with elemental analysis shown in Fig. S3.† Most importantly for the application as a biocide, is that the smaller Ag particles randomly decorate the larger Al2O3 particles.
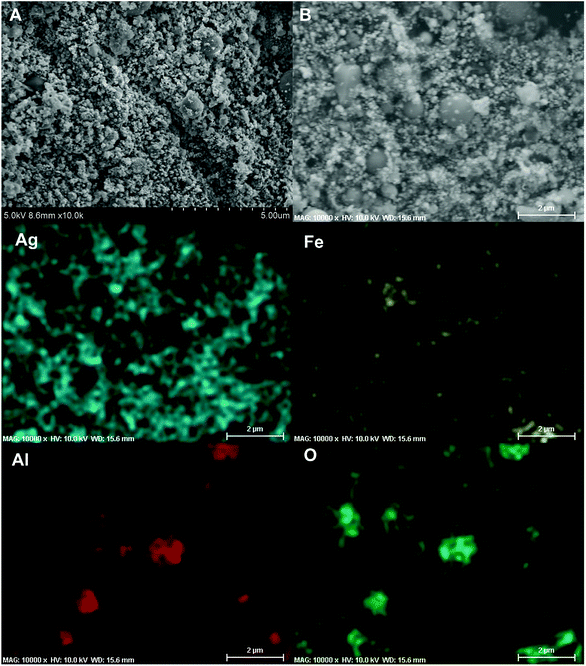 | ||
| Fig. 8 Representative SEM images (A and B) of the Al/AgFeO2 reaction product collected inside the combustion cell; 2D elemental mapping (Ag, Fe, Al and O) of (B) using EDS. | ||
Overall, the intensity of iron signal is the weakest among those four elements under EDS mapping implying the random distribution of iron.
TEM of the reaction product along with an EDS elemental mapping result is shown in Fig. 9. As can be seen, there are two different types of particles similar to the SEM result. One has a spherical shape with a relatively larger particle size and low contrast; another has a spherical/oval shape with smaller size and dark contrast. In the EDS elemental mapping, red represents silver, blue iron, yellow aluminum and green oxygen. Clearly, the three larger spherical particles are Al2O3. Since the mixing of red and blue gives purple, it is reasonable to conclude that the silver and iron positions are mostly overlapped. It could mean a formation of Ag–Fe alloy; however, the SEM results indicate the iron signal is much weaker and always appears with the presence of both aluminum and oxygen signals. To clarify this speculation, an EDS line-scan analysis was employed, and the result will be discussed below. It should also be noted that some white pixels were found in the purple area, implying the presence of oxygen (green plus red and blue equals to white). Thus, those silver- and iron-containing particles might be partially oxidized that could be a result from handling the product in air.
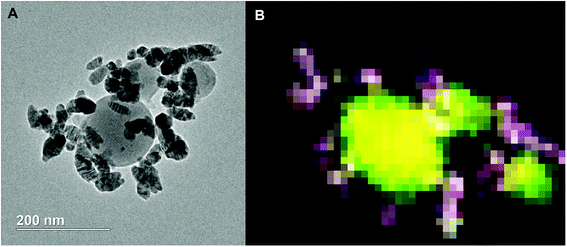 | ||
| Fig. 9 TEM image (A) and 2D elemental mapping (B) of the Al/AgFeO2 reaction product. Red represents Ag, blue represents Fe, yellow represents Al and green represents O. | ||
As we can see from the EDS line-scan result shown in Fig. 10, excerpt for the small oxygen signal peak at ∼0.4 μm, the aluminum and oxygen signals are almost synchronous in positions indicating Al2O3. Like the SEM EDS mapping result, iron is the weakest among all four elements. It overlaps with aluminum and silver, respectively, implying the iron is randomly distributed and excluding the hypothesis of Ag–Fe alloy formation.
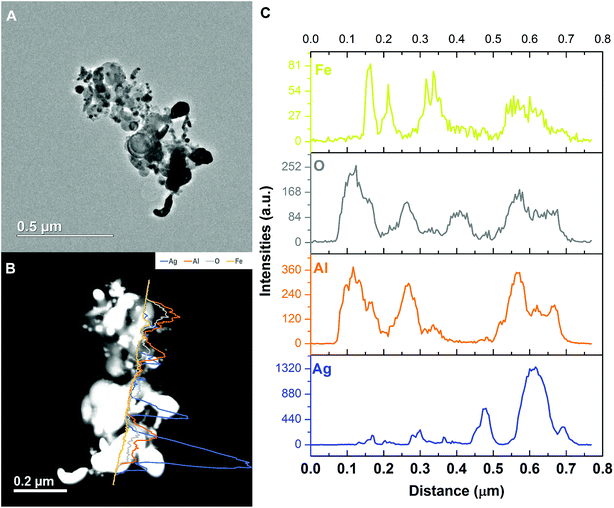 | ||
| Fig. 10 Representative TEM image and the corresponding EDS line-scan data of the Al/AgFeO2 reaction product. | ||
Comparing these two representative TEM images Fig. 9A and 10A, the morphologies of the silver particles are quite different. The ones in Fig. 9A are nanosized with spherical/oval shapes and are decorated on/near the Al2O3 surface that suggests silver product had vaporized and re-condensed.2 This mechanism is beneficial to biocidal applications due to the wide distribution of small sized silver particles during the violent combustion event. As to the one in Fig. 10A, particles at the bottom right with oddly shapes clearly underwent some sintering. Those results are consistent to a previously reported sintering reaction mechanism of Al-based thermites.53,54 However, it seems much less reactants were following the sintering mechanism according to the SEM image shown in Fig. 8 where the majority of silver product has small particle size and distributed randomly.
Conclusions
In this study, the AgFeO2 was prepared via a wet-chemistry method to yield phase pure ∼40 nm particles. The decomposition pathways of AgFeO2 were found to depend on heating rates: decomposition to Ag, O2 and Fe2O3 at ∼600 °C at low heating rate and direct decomposition ∼685 °C at high heating rates.The ignition of Al/AgFeO2 was found to slightly higher than the oxygen release temperature and thus with a similar mechanism to Al/Fe2O3 although ignites at a much lower temperature. Fast video imaging indicates a fine smoke dispersing particle.
Combustion cell test showed that Al/AgFeO2 outperformed other thermites in maximum pressure, pressurization rate and burn time. Moreover, with a pressurization rate three times and 5% less oxygen content of Al/CuO, Al/AgFeO2 yields a comparable result as to Al/hollow-CuO or Al/KClO4/CuO. The fact that Al/AgFeO2 and Al/Ag2O/Fe2O3 share the exact same elemental compositions but feature the highest and lowest pressurization rate, respectively, indicates molecularly incorporation of Ag2O into Fe2O3 outperforms the mechanically mixed Ag2O/Fe2O3 when they were employed as oxidizers in aluminum-based thermites. Post combustion products indicate the formation of elemental silver nanoparticles (∼<80 nm) decorating larger Al2O3 and is this bioavailable.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Support for this work comes from the Defense Threat Reduction Agency.References
- E. R. Blatchley, A. Meeusen, A. I. Aronson and L. Brewster, J. Environ. Eng. Div. (Am. Soc. Civ. Eng.), 2005, 131, 1245–1252 CrossRef CAS.
- K. T. Sullivan, C. W. Wu, N. W. Piekiel, K. Gaskell and M. R. Zachariah, Combust. Flame, 2013, 160, 438–446 CrossRef CAS.
- M. Schoenitz, T. S. Ward and E. L. Dreizin, Proc. Combust. Inst., 2005, 30, 2071–2078 CrossRef.
- T. S. Ward, W. L. Chen, M. Schoenitz, R. N. Dave and E. L. Dreizin, Acta Mater., 2005, 53, 2909–2918 CrossRef CAS.
- K. T. Sullivan, N. W. Piekiel, S. Chowdhury, C. Wu, M. R. Zachariah and C. E. Johnson, Combust. Sci. Technol., 2011, 183, 285–302 CrossRef CAS.
- W. Zhou, M. W. Orr, G. Jian, S. K. Watt, V. T. Lee and M. R. Zachariah, Chem. Eng. J., 2015, 279, 578–588 CrossRef CAS.
- W. B. Zhou, M. W. Orr, V. T. Lee and M. R. Zachariah, Chem. Eng. Sci., 2016, 144, 39–47 CrossRef CAS.
- F. T. Tabit and E. Buys, Int. J. Food Microbiol., 2010, 140, 207–213 CrossRef CAS PubMed.
- S. S. Zhang, C. Badiola, M. Schoenitz and E. L. Dreizin, Combust. Flame, 2012, 159, 1980–1986 CAS.
- S. S. Zhang, M. Schoenitz and E. L. Dreizin, J. Phys. Chem. Solids, 2010, 71, 1213–1220 CrossRef CAS.
- S. S. Zhang, M. Schoenitz and E. L. Dreizin, J. Phys. Chem. C, 2010, 114, 19653–19659 CrossRef CAS.
- H. Wang, G. Jian, W. Zhou, J. B. DeLisio, V. T. Lee and M. R. Zachariah, ACS Appl. Mater. Interfaces, 2015, 7, 17363–17370 CrossRef CAS PubMed.
- E. L. Dreizin, Prog. Energy Combust. Sci., 2009, 35, 141–167 CrossRef CAS.
- C. Rossi, K. Zhang, D. Esteve, P. Alphonse, P. Tailhades and C. Vahlas, J. Microelectromech. Syst., 2007, 16, 919–931 CAS.
- R. A. Yetter, G. A. Risha and S. F. Son, Proc. Combust. Inst., 2009, 32, 1819–1838 CrossRef CAS.
- N. H. Yen and L. Y. Wang, Propellants, Explos., Pyrotech., 2012, 37, 143–155 CrossRef CAS.
- X. Z. Wang, T. Wu and M. R. Zachariah, J. Phys. Chem. C, 2017, 121, 147–152 CrossRef CAS.
- G. Jian, N. W. Piekiel and M. R. Zachariah, J. Phys. Chem. C, 2012, 116, 26881–26887 CrossRef CAS.
- G. Q. Jian, S. Chowdhury, K. Sullivan and M. R. Zachariah, Combust. Flame, 2013, 160, 432–437 CrossRef CAS.
- K. S. Martirosyan, J. Mater. Chem., 2011, 21, 9400–9405 RSC.
- H. Y. Wang, J. B. DeLisio, G. Q. Jian, W. B. Zhou and M. R. Zachariah, Combust. Flame, 2015, 162, 2823–2829 CrossRef CAS.
- X. Z. Wang, W. B. Zhou, J. B. DeLisio, G. C. Egan and M. R. Zachariah, Phys. Chem. Chem. Phys., 2017, 19, 12749–12758 RSC.
- K. Zhang, C. Rossi, G. A. A. Rodriguez, C. Tenailleau and P. Alphonse, Appl. Phys. Lett., 2007, 91, 113117 CrossRef.
- A. Prakash, A. V. McCormick and M. R. Zachariah, Nano Lett., 2005, 5, 1357–1360 CrossRef CAS PubMed.
- G. Q. Jian, L. Liu and M. R. Zachariah, Adv. Funct. Mater., 2013, 23, 1341–1346 CrossRef CAS.
- L. Shen, G. Li, Y. Luo, K. Gao and Z. Ge, Sci. China: Chem., 2014, 57, 797–802 CrossRef CAS.
- G. Jian, S. Chowdhury, J. Feng and M. R. Zachariah, presented in part at the 8th U. S. National Combustion Meeting, Utah, May 19–22, 2013 Search PubMed.
- B. K. Little, E. J. Welle, S. B. Emery, M. B. Bogle, V. L. Ashley, A. M. Schrand and C. M. Lindsay, J. Phys.: Conf. Ser., 2014, 500 Search PubMed.
- T. Wu, A. SyBing, X. Z. Wang and M. R. Zachariah, J. Mater. Res., 2017, 32, 890–896 CrossRef CAS.
- T. Wu, X. Wang, J. B. DeLisio, H. Wang and M. R. Zachariah, Combust. Flame, 2017, 191, 335–342 CrossRef.
- C. Farley and M. Pantoya, J. Therm. Anal. Calorim., 2010, 102, 609–613 CrossRef CAS.
- T. Wu, X. Z. Wang, J. B. DeLisio, S. Holdren and M. R. Zachariah, Carbon, 2018, 130, 410–415 CrossRef CAS.
- W. B. Zhou, J. B. DeLisio, X. Y. Li, L. Liu and M. R. Zachariah, J. Mater. Chem. A, 2015, 3, 11838–11846 RSC.
- M. Comet, G. Vidick, F. Schnell, Y. Suma, B. Baps and D. Spitzer, Angew. Chem., Int. Ed., 2015, 54, 4458–4462 CrossRef CAS PubMed.
- X. L. Hu, W. B. Zhou, X. Z. Wang, T. Wu, J. B. Delisio and M. R. Zachariah, J. Nanopart. Res., 2016, 18, 214 CrossRef.
- X. L. Hu, J. B. DeLisio, X. Y. Li, W. B. Zhou and M. R. Zachariah, Adv. Eng. Mater., 2017, 19, 1500532 CrossRef.
- C. W. Wu, K. Sullivan, S. Chowdhury, G. Q. Jian, L. Zhou and M. R. Zachariah, Adv. Funct. Mater., 2012, 22, 78–85 CrossRef CAS.
- W. B. Zhou, J. B. DeLisio, X. Z. Wang and M. R. Zachariah, Combust. Flame, 2017, 177, 1–9 CrossRef CAS.
- Y. Aly, S. Zhang, M. Schoenitz, V. K. Hoffmann, E. L. Dreizin, M. Yermakov, R. Indugula and S. A. Grinshpun, Combust. Flame, 2014, 161, 303–310 CrossRef CAS.
- C. L. He, J. H. Zhang and J. M. Shreeve, Chem.–Eur. J., 2013, 19, 7503–7509 CrossRef CAS PubMed.
- C. E. Johnson and K. T. Higa, MRS Proc., 2013, 1521 Search PubMed.
- O. Mulamba, E. M. Hunt and M. L. Pantoya, Biotechnol. Bioprocess Eng., 2013, 18, 918–925 CrossRef CAS.
- J. B. DeLisio, X. L. Hu, T. Wu, G. C. Egan, G. Young and M. R. Zachariah, J. Phys. Chem. B, 2016, 120, 5534–5542 CrossRef CAS PubMed.
- T. Wu, P. Y. Zavalij and M. R. Zachariah, Powder Diffr., 2017, 32, 261–264 CrossRef CAS.
- E. Rentz Do Comm Cnmo, J. Nutr. Environ. Med., 2009, 13, 109–118 CrossRef.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- A. B. Smetana, K. J. Klabunde, G. R. Marchin and C. M. Sorensen, Langmuir, 2008, 24, 7457–7464 CrossRef CAS PubMed.
- B. R. Clark and M. L. Pantoya, Phys. Chem. Chem. Phys., 2010, 12, 12653–12657 RSC.
- R. Russell, S. Bless and M. Pantoya, J. Energ. Mater., 2011, 29, 175–192 CrossRef CAS.
- S. H. Fischer and M. C. Grubelich, presented in part at the 24th International Pyrotechnics Seminar, The International Pyrotechnics Society, Monterey, California, USA, 1998 Search PubMed.
- K. Sullivan and M. R. Zachariah, J. Propul. Power, 2010, 26, 467–472 CrossRef CAS.
- L. Zhou, N. Piekiel, S. Chowdhury and M. R. Zachariah, J. Phys. Chem. C, 2010, 114, 14269–14275 CrossRef CAS.
- K. T. Sullivan, W. A. Chiou, R. Fiore and M. R. Zachariah, Appl. Phys. Lett., 2010, 97, 133104 CrossRef.
- K. T. Sullivan, N. W. Piekiel, C. Wu, S. Chowdhury, S. T. Kelly, T. C. Hufnagel, K. Fezzaa and M. R. Zachariah, Combust. Flame, 2012, 159, 2–15 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08997c |
| This journal is © The Royal Society of Chemistry 2019 |