Open Access Article
Open Access ArticleMicrostructure and hydrogen storage kinetics of Mg89RE11 (RE = Pr, Nd, Sm) binary alloys
Li Chen *ab,
Changyi Hua and
Feng Liub
*ab,
Changyi Hua and
Feng Liub
aKunming University of Science and Technology, Kunming 650093, China
bKunming Institute of Precious Metals, Kunming 650106, China. E-mail: 669282531@qq.com
First published on 5th February 2019
Abstract
The present work reports the fabrication of Mg89RE11 (RE = Pr, Sm, Nd) alloys by a vacuum induction furnace. The phase analysis, structure characterization and microstructure observation of Mg89RE11 alloys were carried out by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). There exhibits a multiphase microstructure in the as-cast Mg89RE11 (RE = Pr, Nd, Sm) alloys from the comprehensive analysis made from XRD and SEM, which are containing the major phase (RE5Mg41) and several secondary phases (REMg3 and REMg12). The detections of XRD and TEM reveal that these experimental alloys turn into a MgH2 nanocrystalline composite with equably distributed RE hydride nanoparticles after hydriding and this MgH2 major phase turns into a Mg nanocrystalline after dehydriding. The determination results of the hydrogen storage kinetics show that adding the rare earth element (Pr, Sm and Nd) ameliorates the hydriding and dehydriding kinetics of the Mg-based alloys dramatically. The hydrogen desorption activation energy Ea(de) of Mg89Pr11, Mg89Nd11, Mg89Sm11 are 140.595, 139.191, 135.280 kJ mol−1 H2, respectively. Specially, the hydrogen storage capacity (wt%) of Mg89Sm11 alloy that added Sm element can reached 5 wt%. The improvement of the hydrogen storage performance of Mg89RE11 alloys can be principally ascribed to the RE hydride nanoparticles facilitating the hydriding and dehydriding reactions.
Introduction
Mg-based alloys have been widely deemed as attractive hydrogen storage materials for hydrogen fuel cells due to their outstanding advantages including good hydrogen storage capacity, low-cost and high accessibility.1,2 However, the application of Mg-based alloys has been hampered by their relatively high dehydrogenation temperature and sluggish hydriding/dehydriding kinetics all along.3,4 Among the methods to improve the hydriding/dehydriding kinetics, finding effective alloying elements has been one of the most important research focuses for Mg-based alloys.So far, a variety of chemical elements, such as In,5–7 Ni,8–10 Zr,11,12 Ag,13 Al,14,15 Ti,16,17 rare earth (RE) elements18 and their composites,19–23 have been exploited as the alloying elements for the Mg-based alloys. Among them, adding RE elements attracts extensive interest for the Mg-based alloys due to its excellent improvement of the hydriding/dehydriding rate, which may be a better choice to meet the requirements of future hydrogen energy storage systems.24–29 Therefore, the addition of rare earth elements into Mg-based alloys as alloying elements shed light on the better hydrogen storage properties for alloys. The hydrogen storage capacity of La2Mg17 reaches 6 wt% at the temperature of 350 °C.30 The Mg3La compound prepared by induction melting can absorb 2.89 wt% of hydrogen reversibly at 296 °C.31 Wu et al.32 investigated the hydrogen storage properties and phase transitions of Mg17Ba2, which has reversible hydrogen capacity of 4.0 wt% H2. Ma et al.33,34 proposed the addition of Ni to the CaMg2-based alloys resulted in room-temperature hydrogen absorption without an activation process, and a maximum hydrogen-absorption capacity of 5.65 wt%. Moreover, dual-tuning effects of the thermodynamics and kinetics for Mg-based materials is one of the key issues for hydrogen economy and hydrogen storage materials.35–37 Cao et al.35 found that the Mg85In5Al5Ti5 alloy displays both enhanced dehydriding kinetics and thermodynamics, which is prepared by plasma milling (P-milling).36 The addition of In into Mg2Ni not only greatly enhanced the dehydriding kinetics but also significantly lowered the thermodynamic stability.37
Although the research of Mg-based hydrogen storage alloys has made much substantial progress, the cost of hydrogen storage alloys has been increased by high alloying element content, and the hydrogen storage performance needs to be further improved. In this work, we report a time-saving vacuum inducing smelting method to synthesize low alloying Mg89RE11 (RE = Pr, Nd, Sm) binary alloys as new hydrogen storage materials. On the one hand, we evaluated hydrogen storage properties of the Mg89RE11 (RE = Pr, Nd, Sm) binary alloys. On the other hand, we deeply discussed the hydriding and dehydriding mechanism for the Mg89RE11 (RE = Pr, Nd, Sm) binary alloys further.
Experimental
The compositions of alloys in this experiment were designed to be Mg89RE11 (RE = Pr, Nd, Sm). The alloy ingots were melted and synthesized with a certain mass ratio metal by using a vacuum induction furnace. In order to prevent the volatilization and oxidation of magnesium and rare earth elements, 0.04 MPa helium was used as the protection gas in the smelting process. After the liquid alloy is injected into the copper mold, the parent alloy ingot is obtained after cooling.Some microstructures of the samples, including microscopic morphology, phase composition and crystal structure, were observed and characterized by TEM (JEM-2100F) linked with an SAED (selected area electron diffraction), SEM (FEI Quanta 400) linked with an EDS (Energy Disperse Spectroscopy, EDAX Apollo 40 silicon drift detector). For phase analysis, XRD (D/max/2400) technique was employed.
A series of tests on the kinetics of hydrogen absorption and desorption of alloys were carried out on an automatically controlled Sieverts apparatus. The alloys used in above test need to be mechanically pulverized in advance and the powder between 48–75 μm is screened for testing. The sample weighing 1 g was put into the reaction chamber and repeatedly absorbed/desorbed hydrogen until completely activated. When the initial hydrogen pressure of hydriding process was set at 3 MPa, the hydrogen absorption experiment was carried out in the temperature range of 150–380 °C. At the same temperature, the initial hydrogen pressure in hydrogen desorption was set at 1 × 10−4 MPa.
Results and discussion
Microstructure of as-cast Mg89RE11 (RE = Pr, Nd, Sm) binary alloys
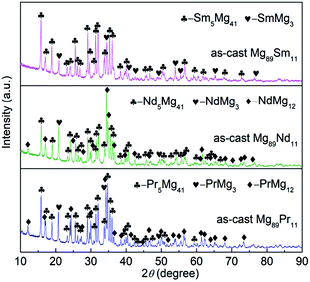
The phase composition and phase structure of the as-cast Mg89RE11 (RE = Pr, Nd, Sm) binary alloys are shown contrastively in the XRD diagram of Fig. 1. These three alloys have the typical crystalline structure. After the analysis using the Jade 6.0 software on the crystal cell parameters of the phase of as-cast alloys, we could find the as-cast Mg89Pr11 alloy is composed of major phase Pr5Mg41 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 1.4780 nm, c = 1.0430 nm) and secondary phase PrMg3 with space group of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) m cubic structure (the lattice parameter is showing a = 0.7388 nm) and PrMg12 with space group of I4mmm tetragonal structure (the lattice parameters are showing a = b = 1.0340 nm, c = 0.5980 nm). The as-cast Mg89Nd11 alloy contains major phase Nd5Mg41 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 1.4760 nm, c = 1.0390 nm) and secondary phase NdMg3 with space group of Fm
m cubic structure (the lattice parameter is showing a = 0.7388 nm) and PrMg12 with space group of I4mmm tetragonal structure (the lattice parameters are showing a = b = 1.0340 nm, c = 0.5980 nm). The as-cast Mg89Nd11 alloy contains major phase Nd5Mg41 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 1.4760 nm, c = 1.0390 nm) and secondary phase NdMg3 with space group of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) m cubic structure (the lattice parameter is showing a = 0.7438 nm) and NdMg12 with space group of I4mmm tetragonal structure (the lattice parameters are showing a = b = 1.0310 nm, c = 0.5930 nm). The as-cast Mg89Sm11 alloy is made up with two parts, major phase Sm5Mg41 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 1.4815 nm, c = 1.0366 nm) and secondary phase SmMg3 with space group of Fm
m cubic structure (the lattice parameter is showing a = 0.7438 nm) and NdMg12 with space group of I4mmm tetragonal structure (the lattice parameters are showing a = b = 1.0310 nm, c = 0.5930 nm). The as-cast Mg89Sm11 alloy is made up with two parts, major phase Sm5Mg41 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 1.4815 nm, c = 1.0366 nm) and secondary phase SmMg3 with space group of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) m cubic structure (the lattice parameter is showing a = 0.7346 nm). Unlike the Mg89Sm11 alloy, both Mg89Pr11 alloy and Mg89Nd11 alloy include three different types of phases. The stronger the secondary phase diffraction peaks of Mg89Pr11 alloy and Mg89Nd11 alloy express, the higher the secondary phase content will be.
m cubic structure (the lattice parameter is showing a = 0.7346 nm). Unlike the Mg89Sm11 alloy, both Mg89Pr11 alloy and Mg89Nd11 alloy include three different types of phases. The stronger the secondary phase diffraction peaks of Mg89Pr11 alloy and Mg89Nd11 alloy express, the higher the secondary phase content will be.
Fig. 2 displays the SEM micrographs of as-cast Mg89RE11 (RE = Pr, Nd, Sm) binary alloys. Taking as-cast Mg89Sm11 alloy as an example, the EDS patterns of A and B regions are depicted to represent the alloy content of each region. The two regions with different degrees of darkness in the as-cast alloy represent the two-phase structure of the alloy. The results combining the EDS and XRD analysis show that the white and dark gray areas are SmMg3 and Sm5Mg41, respectively.
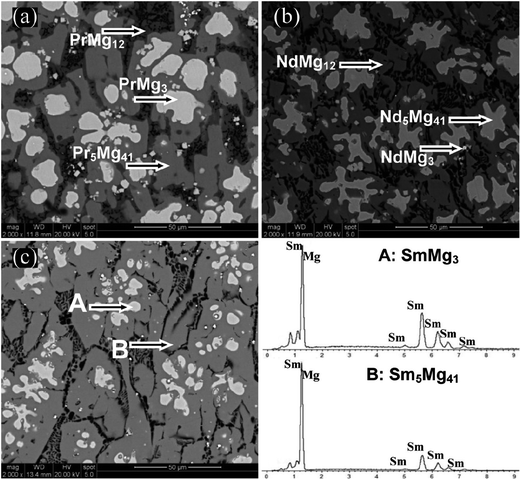 | ||
| Fig. 2 SEM micrographs and EDS of as-cast samples for Mg89RE11 (RE = Pr, Nd, Sm) alloys: (a) RE = Pr; (b) RE = Nd; (c) RE = Sm. | ||
Hydriding and dehydriding mechanism
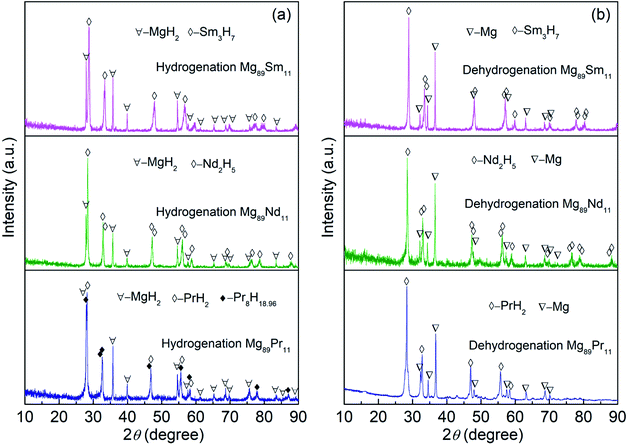
The XRD patterns of the Mg89RE11 (RE = Pr, Nd, Sm) binary alloys after hydrogen absorption/desorption completely are shown in Fig. 3, all of which alloys display the typical crystalline structure. For Mg89Pr11 alloy, there are three phases formed after hydrogen absorption, including the major phase MgH2 with space group of P42/mnm tetragonal structure (the lattice parameters are showing a = b = 0.4517 nm, c = 0.3020 nm) and secondary phase PrH2 with space group of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) m cubic structure (the lattice parameter is showing a = 0.5486 nm) and Pr8H18.96 with space group of I41md tetragonal structure (the lattice parameters are showing a = b = 0.5494 nm, c = 1.1078 nm). While after hydrogen desorption, the major phase Mg with space group of P63/mmc hexagonal structure (the lattice parameters are showing a = b = 0.3209 nm, c = 0.1624 nm) and secondary phase PrH2 are existing. With respect to Mg89Nd11, the major phase MgH2 and secondary phase Nd2H5 with space group of I41md tetragonal structure (the lattice parameters are showing a = b = 0.5413 nm, c = 1.0870 nm) exist after hydrogen absorption. And the major phase Mg and secondary phase Nd2H5 form after hydrogen desorption. With regard to Mg89Sm11, the major phase MgH2 and secondary phase Sm3H7 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 0.3778 nm, c = 0.5365 nm) form after hydrogen absorption. It is the major phase Mg and secondary phase Sm3H7 that are existing after hydrogen desorption. It can be concluded that the hydride PrH2, Nd2H5 and Sm3H7 possess characteristics of good thermal stability at 400 °C.38 According to the XRD analysis and literature,38 we infer conceivable reaction pathways:
m cubic structure (the lattice parameter is showing a = 0.5486 nm) and Pr8H18.96 with space group of I41md tetragonal structure (the lattice parameters are showing a = b = 0.5494 nm, c = 1.1078 nm). While after hydrogen desorption, the major phase Mg with space group of P63/mmc hexagonal structure (the lattice parameters are showing a = b = 0.3209 nm, c = 0.1624 nm) and secondary phase PrH2 are existing. With respect to Mg89Nd11, the major phase MgH2 and secondary phase Nd2H5 with space group of I41md tetragonal structure (the lattice parameters are showing a = b = 0.5413 nm, c = 1.0870 nm) exist after hydrogen absorption. And the major phase Mg and secondary phase Nd2H5 form after hydrogen desorption. With regard to Mg89Sm11, the major phase MgH2 and secondary phase Sm3H7 with space group of I4m tetragonal structure (the lattice parameters are showing a = b = 0.3778 nm, c = 0.5365 nm) form after hydrogen absorption. It is the major phase Mg and secondary phase Sm3H7 that are existing after hydrogen desorption. It can be concluded that the hydride PrH2, Nd2H5 and Sm3H7 possess characteristics of good thermal stability at 400 °C.38 According to the XRD analysis and literature,38 we infer conceivable reaction pathways:| Mg89Pr11 + H2 → PrH2 + Pr8H18.96 + MgH2 ↔ PrH2 + Mg + H2 | (1) |
| Mg89Nd11 + H2 →Nd2H5 + MgH2 ↔ Nd2H5 + Mg + H2 | (2) |
| Mg89Sm11 + H2 → Sm3H7 + MgH2 ↔ Sm3H7 + Mg + H2 | (3) |
The fundamental reason for reversible hydrogen absorption/desorption of the active Mg89RE11 (RE = Pr, Nd, Sm) binary alloys is coming from the formation and decomposition of MgH2.
Fig. 4 shows the TEM and HRTEM micrographs and SAED rings of the fully hydrogen desorption samples at 380 °C for Mg89RE11 (RE = Pr, Nd, Sm) alloy. According to the inset of Fig. 4(a)–(c), the alloys are composed of the Mg and REHx (PrH2, Nd2H5 and Sm3H7) nanocrystal phases, which conforms to the XRD detections. As the observation of the internal lattice structure from HRTEM (Fig. 4(d)), the interplanar spacing of Mg nanocrystalline on (100) crystal surface is 0.278 nm and the secondary phase Sm3H7 nanocrystal on (101) crystal surface is 0.309 nm. The hydrogen absorption alloy turns into a Mg nanocrystalline composite with equably distributed Sm3H7 nanoparticles (<100 nm) after dehydriding. The mechanism of the nucleation and growth of the formed REHx (PrH2, Nd2H5 and Sm3H7) composites is similar to CeH2.73–MgH2–Ni composites.39 It can be found that the secondary phase PrH2, Pr8H18.96, Nd2H5 and Sm3H7 served as catalysts39 affect the hydrogen absorption and desorption rates.
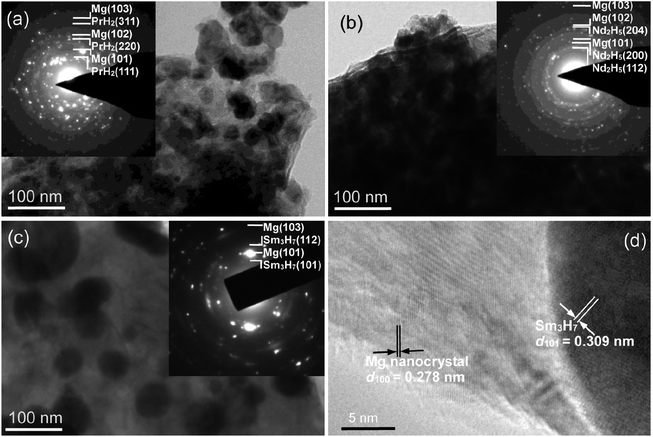 | ||
| Fig. 4 TEM and HRTEM micrographs and SAED rings of the hydrogen desorption samples for Mg89RE11 (RE = Pr, Nd, Sm) alloy: (a) RE = Pr; (b) RE = Nd; (c) and (d) RE = Sm. | ||
Hydrogen storage kinetics
For studying the effects of the alloying of Mg with rare earth elements on hydrogen storage kinetics, it is necessary to determine the hydrogen absorption/desorption curves of these Mg89RE11 (RE = Pr, Nd, Sm) binary alloys at various temperatures. The Johnson–Mehl–Avrami–Kolmogorov (JMAK) method can be generally applied to model the hydrogen absorption and desorption data, and there is a linear equation expressed following:40
ln[−ln(1 − α)] = η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + η k + η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t
| (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t and obtain the η and η
t and obtain the η and η![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values at various temperatures lightly from the slope and intercept of above plots. As a result, the rate constant k will be figured out expediently using eqn (4). Thus, by means of introducing the k values into the following Arrhenius equation, we will calculate the apparent activation energy (Ea) for the absorption and desorption process logically:41
k values at various temperatures lightly from the slope and intercept of above plots. As a result, the rate constant k will be figured out expediently using eqn (4). Thus, by means of introducing the k values into the following Arrhenius equation, we will calculate the apparent activation energy (Ea) for the absorption and desorption process logically:41
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−Ea/RT] exp[−Ea/RT]
| (5) |
Fig. 5(a) shows the hydrogen absorption curves of the Mg89Pr11 alloy, which were measured at 150 to 380 °C under the initial hydrogen pressure of 3 MPa. The increase of hydrogen absorption capacity will bring with the increase of the temperature for the alloy. It can be suggested that increasing temperature facilitates the improvement of the hydriding kinetics of the alloy. In terms of specific data, the hydrogen absorption capacity of 3.712 wt% for the alloy can be reached within 10 min at 250 °C, compared to only 2.4 wt% for pure MgH2 even if it lasts 20 min and under the same condition.42 Fig. 5(b) displays the hydrogen desorption curves of the Mg89Pr11 alloy, which were measured at 300 to 360 °C under 1 × 10−4 MPa pressure. The final hydrogen desorption capacity of 4.7 wt% for this alloy is lower than own hydrogen absorption capacity due to the lower final hydrogen pressure (<1 × 10−2 MPa) of the test system. The hydrogen desorption of the hydrogenated alloy at 320 °C, 340 °C and 360 °C have finished within 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 s, 3195 s and 2025 s, respectively. Compared to no decomposition for the milled MgH2 at 300 °C, 3.15 wt% H for the Mg89Pr11 alloy is desorbed in 200 min, suggesting that this alloy displays a good desorption kinetics. Fig. 5(c) and (d) illustrate the Arrhenius plot of ln
350 s, 3195 s and 2025 s, respectively. Compared to no decomposition for the milled MgH2 at 300 °C, 3.15 wt% H for the Mg89Pr11 alloy is desorbed in 200 min, suggesting that this alloy displays a good desorption kinetics. Fig. 5(c) and (d) illustrate the Arrhenius plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1000/RT under hydrogenation and dehydrogenation conditions, respectively. The calculated activation energy for the hydrogenation (Ea(ab) = 90.35 kJ mol−1) is much lower than that of Mg powder after cyclic activation process (100 kJ mol−1).43 For the dehydrogenation, the values of activation energy are 160 and 156 kJ mol−1 for un-milled MgH2 (ref. 44 and 45) and the value of which is 158.5 kJ mol−1 for pure milled MgH2,46 which values all show larger than the calculated Ea(de) value (140.60 kJ mol−1) for the Mg89Pr11 alloy.
k vs. 1000/RT under hydrogenation and dehydrogenation conditions, respectively. The calculated activation energy for the hydrogenation (Ea(ab) = 90.35 kJ mol−1) is much lower than that of Mg powder after cyclic activation process (100 kJ mol−1).43 For the dehydrogenation, the values of activation energy are 160 and 156 kJ mol−1 for un-milled MgH2 (ref. 44 and 45) and the value of which is 158.5 kJ mol−1 for pure milled MgH2,46 which values all show larger than the calculated Ea(de) value (140.60 kJ mol−1) for the Mg89Pr11 alloy.
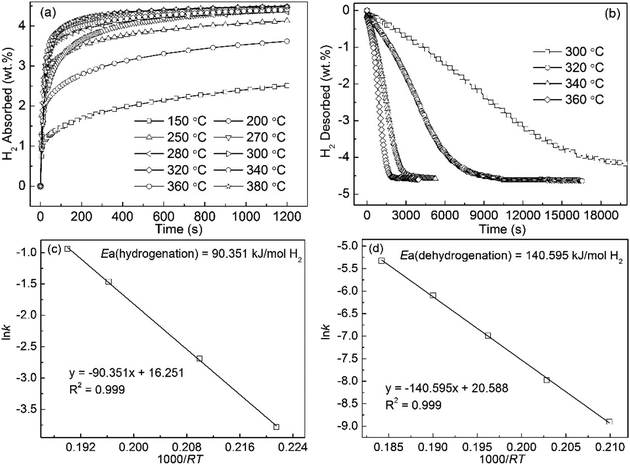 | ||
| Fig. 5 Isothermal hydrogen absorption (a) and desorption (b) kinetic curves of Pr11Mg89 alloy at different temperatures and the corresponding Arrhenius plots ((c) and (d)). | ||
In the initial pressure in the 3 Mpa, the hydrogen absorption curves of the Mg89Nd11 alloy tested at temperatures ranging from 150 to 380 °C are displayed in Fig. 6(a). The hydrogen absorption capacity of the alloy increases from 2.403 wt% at 150 °C to 4.491 wt% at 380 °C. It can be found that the increase of the temperature facilitates the improvement of the hydriding kinetics of the alloy. Under the condition of 250 °C, the hydrogen absorption capacity of 3.712 wt% for the alloy could reach within 10 min. Fig. 6(b) exhibits the hydrogen desorption curves of the Mg89Nd11 alloy measured at 300 to 360 °C under 1 × 10−4 MPa pressure. The final hydrogen desorption capacity of 4.7 wt% for the alloy is also lower than the hydrogen absorption capacity. The hydrogen desorption of the hydrogenated alloy at 320 °C, 340 °C and 360 °C have finished within 8235 s, 3420 s and 1890 s, respectively. Unlike no decomposition for the milled MgH2 at 300 °C, 3.45 wt% H for the Mg89Nd11 alloy is desorbed in 200 min. Fig. 6(c) and (d) illustrate the Arrhenius plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1000/RT that is able to reflect the hydrogenation and dehydrogenation kinetics of the Mg89Nd11 alloy. The calculated Ea(ab) value (84.072 kJ mol−1) is also much lower than that of Mg powder (100 kJ mol−1). For the dehydrogenation, the Ea(de) value from calculating is 139.193 kJ mol−1 approximately.
k vs. 1000/RT that is able to reflect the hydrogenation and dehydrogenation kinetics of the Mg89Nd11 alloy. The calculated Ea(ab) value (84.072 kJ mol−1) is also much lower than that of Mg powder (100 kJ mol−1). For the dehydrogenation, the Ea(de) value from calculating is 139.193 kJ mol−1 approximately.
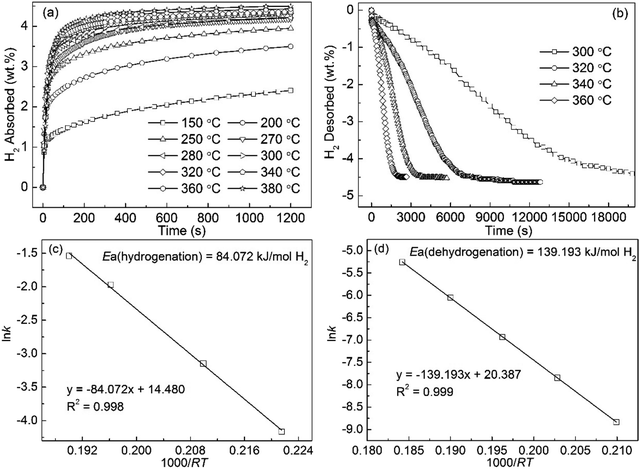 | ||
| Fig. 6 Isothermal hydrogen absorption (a) and desorption (b) kinetic curves of Nd11Mg89 alloy at different temperatures and the corresponding Arrhenius plots ((c) and (d)). | ||
The hydrogen absorption curves of the Mg89Sm11 alloy, which were measured at 150 to 380 °C under 3 MPa hydrogen pressure, shows in Fig. 7(a). The hydrogen absorption capacity of the alloy increases from 2.803 wt% at 150 °C to 5.022 wt% at 380 °C, suggesting that the increasing temperature promotes the improvement of the hydriding kinetics. The hydrogen absorption capacity of 4.112 wt% for the alloy can achieve within 10 min at 250 °C. The hydrogen desorption curves of the Mg89Sm11 alloy tested at 300 to 360 °C under 1 × 10−4 MPa are depicted in Fig. 7(b). The final hydrogen desorption capacity of 5.0 wt% for the alloy is lower than the hydrogen absorption capacity. Length of time for the completion of hydrogen desorption of the hydrogenated alloy at 320 °C, 340 °C and 360 °C have finished within 6556 s, 2527 s and 1106 s, respectively. Compared to no decomposition for the milled MgH2 at 300 °C, 3.572 wt% H for the Mg89Sm11 alloy is desorbed in 200 min. The Arrhenius plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1000/RT for the hydrogenation and dehydrogenation kinetics are displayed in Fig. 7(c) and (d). The calculated Ea(ab) and Ea(de) values are about 77.802 kJ mol−1 135.280 kJ mol−1, respectively.
k vs. 1000/RT for the hydrogenation and dehydrogenation kinetics are displayed in Fig. 7(c) and (d). The calculated Ea(ab) and Ea(de) values are about 77.802 kJ mol−1 135.280 kJ mol−1, respectively.
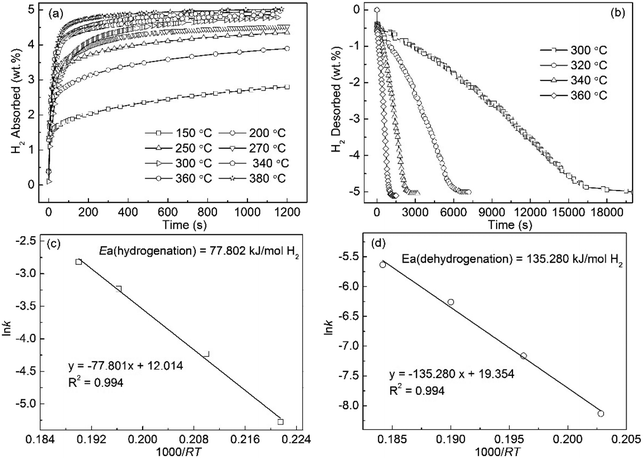 | ||
| Fig. 7 Isothermal hydrogen absorption (a) and desorption (b) kinetic curves of Sm11Mg89 alloy at different temperatures and the corresponding Arrhenius plots ((c) and (d)). | ||
Synthesizing the above analysis, the hydrogen storage kinetics properties of Mg89RE11 (RE = Pr, Nd, Sm) alloys are listed in the Table 1. For Mg89RE11 (RE = Pr, Nd, Sm) alloys, the order of hydrogen absorption and desorption rate is Sm > Nd > Pr. However, the apparent activation energy (Ea) both absorption and desorption course are all decreased, and the order is Sm < Nd < Pr. It can be concluded that the Mg89Sm11 alloy is the highest hydrogen storage kinetics properties alloy. The results shows that the hydrogen sorption properties of Mg-based alloys can be substantially improved by forming composites having catalytic effect and proper microstructure features.47–49
| Mg89RE11 alloy | 250 °C, capacity within 10 min | 320 °C, complete dehydrogenation time | Ea(ab) kJ mol−1 | Ea(de) kJ mol−1 |
|---|---|---|---|---|
| RE = Pr | 3.712 wt% | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 s 350 s |
90.35 | 140.60 |
| RE = Nd | 3.895 wt% | 8235 s | 84.07 | 139.19 |
| RE = Sm | 4.112 wt% | 6556 s | 77.802 | 135.28 |
| MgH2 (ref. 42–45) | 2.4 wt% | Stable | 100 | 156/160 |
Conclusions
In summary, Mg89RE11 (RE = Pr, Nd, Sm) alloys can be synthesized via a time-saving vacuum inducing smelting approach. The conclusions drawn from this work is sketched as blows:(1) The as-cast Mg89RE11 (RE = Pr, Nd, Sm) alloy contains a major phase RE5Mg41 (RE = Pr, Nd, Sm) as well as two kinds of secondary phase REMg3 (RE = Pr, Nd, Sm) or REMg12 (RE = Pr, Nd). After hydrogen absorption, there will form a major phase MgH2 and a secondary phase PrH2 and Pr8H18.96 for RE = Pr, Nd2H5 for RE = Nd, Sm3H7 for RE = Sm. After hydrogen desorption, there will exist the major phase Mg and a secondary phase PrH2 for RE = Pr, Nd2H5 for RE = Nd, Sm3H7 for RE = Nd.
(2) The RE hydrides (PrH2, Nd2H5 and Sm3H7) are equably distributed in the MgH2 nanocrystalline phase and possess characteristics of good thermal stability. These RE hydrides (<100 nm) served as catalysts increase the hydrogen absorption and desorption rates. In these three kinds of hydrogen storage alloys, the Mg89Sm11 alloy shows the lowest apparent activation energy both in the absorption and desorption and the fastest hydrogen absorption and desorption rates.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Key R&D Program of China (2016YFB0101309).References
- K. F. A. Zinsou and J. R. A. Fernández, Energy Environ. Sci., 2010, 3, 497–676 RSC.
- U. D. Rafi, X. Qu and P. Li, RSC Adv., 2012, 2, 4891–4903 RSC.
- N. Juahir, N. S. Mustafa and A. M. Sinin, RSC Adv., 2015, 5, 60983–60989 RSC.
- H. Liu, C. Wu and H. Zhou, RSC Adv., 2015, 5, 22091–22096 RSC.
- Y. S. Lu, M. Zhu, H. Wang, Z. M. Li, L. Z. Ouyang and J. W. Liu, Int. J. Hydrogen Energy, 2014, 39, 14033–14038 CrossRef CAS.
- Z. J. Cao, L. Z. Ouyang, Y. Y. Wu, H. Wang, J. W. Liu, F. Fang, D. L. Sun, Q. G. Zhang and M. Zhu, J. Alloys Compd., 2015, 623, 354–358 CrossRef CAS.
- L. Z. Ouyang, Z. J. Cao, H. Wang, J. W. Liu, D. L. Sun, Q. A. Zhang and M. Zhu, J. Alloys Compd., 2014, 586, 113–117 CrossRef CAS.
- S. Løken, J. K. Solberg, J. P. Maehlen, R. V. Denys, M. V. Lototsky and B. P. Tarasov, J. Alloys Compd., 2007, 446–447, 114–120 CrossRef.
- Y. Wu, M. V. Lototsky, J. K. Solberg, V. A. Yartys, W. Han and S. X. Zhou, J. Alloys Compd., 2009, 477, 262–266 CrossRef CAS.
- J. L. Bobet, E. Akiba and B. Darriet, Int. J. Hydrogen Energy, 2001, 26, 493–501 CrossRef CAS.
- R. R. Shahi, A. Bhatanagar, S. K. Pandey, V. Shukla, T. P. Yadav, M. A. Shaz and O. N. Srivastava, Int. J. Hydrogen Energy, 2015, 40, 11506–11513 CrossRef CAS.
- B. Molinas, A. A. Ghilarducci, M. Melnichuk, H. L. Corso, H. A. Peretti and F. Agresti, Int. J. Hydrogen Energy, 2009, 34, 4597–4601 CrossRef CAS.
- L. Z. Ouyang, Z. J. Cao, L. Yao, H. Wang, J. W. Liu and M. Zhu, Int. J. Hydrogen Energy, 2014, 39, 13616–13621 CrossRef CAS.
- L. Z. Ouyang, Z. J. Cao, L. L. Li, H. Wang, J. W. Liu, D. Min, Y. W. Chen, F. M. Xiao, R. H. Tang and M. Zhu, Int. J. Hydrogen Energy, 2014, 39, 12765–12772 CrossRef CAS.
- B. P. Mamula, J. G. Novaković, I. Radisavljević, N. Ivanović and N. Novaković, Int. J. Hydrogen Energy, 2014, 39, 5874–5887 CrossRef.
- G. Liang, J. Huot, S. Boily, A. V. Neste and R. Schulz, J. Alloys Compd., 1999, 292, 247–252 CrossRef CAS.
- Z. Dehouche, J. Goyette, T. K. Bose and R. Schulz, Int. J. Hydrogen Energy, 2003, 28, 983–988 CrossRef CAS.
- S. Kalinichenka, L. Röntzsch, T. Riedl, T. Weißgärber and B. Kieback, Int. J. Hydrogen Energy, 2011, 36, 10808–10815 CrossRef CAS.
- P. Aep, T. Akito and J. S. Szmyd, Int. J. Hydrogen Energy, 2009, 34, 3032–3037 CrossRef.
- H. Y. Leng, Y. B. Pan, Q. Li and K. C. Chou, Int. J. Hydrogen Energy, 2014, 39, 13622–13627 CrossRef CAS.
- S. Hamed, A. Kaflou and A. Simch, Int. J. Hydrogen Energy, 2009, 34, 7724–7730 CrossRef.
- R. Vijay, R. Sundaresan, M. P. Maiya and M. S. Srinivasa, Int. J. Hydrogen Energy, 2007, 32, 2390–2399 CrossRef CAS.
- G. Liang, J. Huot, S. Boily and A. V. Neste, J. Alloys Compd., 2000, 297, 267–275 CrossRef.
- Z. M. Yuan, T. Yang, W. G. Bu, H. W. Shang, Y. Qi and Y. H. Zhang, Int. J. Hydrogen Energy, 2016, 41, 5994–6003 CrossRef CAS.
- Z. M. Yuan, Y. H. Zhang, T. Yang, W. G. Bu, S. H. Guo and D. L. Zhao, Renewable Energy, 2018, 116, 878–891 CrossRef CAS.
- Z. M. Yuan, W. Zhang, P. L. Zhang, Y. H. Zhang, W. G. Bu, S. H. Guo and D. L. Zhao, RSC Adv., 2017, 7, 56365–56374 RSC.
- Z. M. Yuan, J. He, L. Yang, Z. J. Xia, D. L. Zhao, C. Y. You and W. J. Ren, J. Appl. Phys., 2015, 117, 17D104 CrossRef.
- Z. M. Yuan, W. Zhang, Y. H. Zhang, S. H. Guo, X. P. Dong and D. L. Zhao, J. Mater. Sci. Technol., 2018, 34, 1851–1858 CrossRef.
- Y. H. Zhang, Z. M. Yuan, T. Yang, D. C. Feng, Y. Cai and D. L. Zhao, J. Alloys Compd., 2016, 688, 585–593 CrossRef CAS.
- S. Yajima and H. K. Toma, J. Less-Common Met., 1977, 44, 301–306 Search PubMed.
- L. Z. Ouyang, F. X. Qin and M. Zhu, Scr. Mater., 2006, 55, 1075–1078 CrossRef CAS.
- D. Wu, L. Ouyang, C. Wu, Q. Gu, H. Wang, J. Liu and M. Zhu, J. Alloys Compd., 2017, 690, 519–522 CrossRef CAS.
- M. Ma, R. Duan, L. Ouyang, X. Zhu, Z. Chen, C. Peng and M. Zhu, J. Alloys Compd., 2017, 691, 929–935 CrossRef CAS.
- M. Ma, R. Duan, L. Ouyang, X. Zhu, C. Peng and M. Zhu, Int. J. Hydrogen Energy, 2017, 42, 22312–22317 CrossRef CAS.
- Z. Cao, L. Ouyang, Y. Wu, H. Wang, J. Liu, F. Fang, D. Sun, Q. Zhang and M. Zhu, J. Alloys Compd., 2015, 623, 354–358 CrossRef CAS.
- L. Ouyang, Z. Cao, H. Wang, R. Hu and M. Zhu, J. Alloys Compd., 2017, 691, 422–435 CrossRef CAS.
- L. Z. Ouyang, Z. J. Cao, H. Wang, J. W. Liu, D. L. Sun, Q. A. Zhang and M. Zhu, Int. J. Hydrogen Energy, 2013, 38, 8881–8887 CrossRef CAS.
- V. A. Yartys, O. Gutfleisch, V. V. Panasyuk and I. RHarris, J. Alloys Compd., 1997, 253–254, 128–133 CrossRef CAS.
- L. Z. Ouyang, X. S. Yang, M. Zhu, J. W. Liu, H. W. Dong and D. L. Sun, J. Phys. Chem. C, 2014, 118, 7808–7820 CrossRef CAS.
- T. Sadhasivam, M. S. L. Hudson, S. K. Pandey, A. Bhatnagar, M. K. Singh, K. Gurunathan and O. N. Srivastava, Int. J. Hydrogen Energy, 2013, 38, 7353–7362 CrossRef CAS.
- T. Liu, Y. R. Cao, C. G. Qin, W. S. Chou and X. G. Li, J. Power Sources, 2014, 246, 277–282 CrossRef CAS.
- R. K. Singh, T. Sadhasivam, G. I. Sheeja, P. Singh and O. N. Srivastava, Int. J. Hydrogen Energy, 2013, 38, 6221–6225 CrossRef CAS.
- T. R. Jensen, A. Andreasen, T. Vegge, J. W. Andreasen, K. Ståhl and A. S. Pedersen, Int. J. Hydrogen Energy, 2006, 31, 2052–2062 CrossRef CAS.
- J. Huot, G. Liang, S. Boily, A. V. Neste and R. Schulz, J. Alloys Compd., 1999, 293–295, 495–500 CrossRef CAS.
- J. F. Fernandez and C. R. Sanchez, J. Alloys Compd., 2002, 340, 189–199 CrossRef CAS.
- J. F. Mao, Z. P. Guo, X. B. Yu, H. K. Liu, Z. Wu and J. Ni, Int. J. Hydrogen Energy, 2010, 35, 4569–4575 CrossRef CAS.
- M. Zhu, H. Wang, L. Z. Ouyang and M. Q. Zeng, Int. J. Hydrogen Energy, 2006, 31, 251–257 CrossRef CAS.
- J. Cui, H. Wang, J. Liu, L. Ouyang, Q. Zhang, D. Sun, X. Yao and M. Zhu, J. Mater. Chem. A, 2013, 1, 5603–5611 RSC.
- Y. Yin, B. Li, Z. Yuan, Y. Qi and Y. Zhang, RSC Adv., 2018, 8, 34525–34535 RSC.
| This journal is © The Royal Society of Chemistry 2019 |