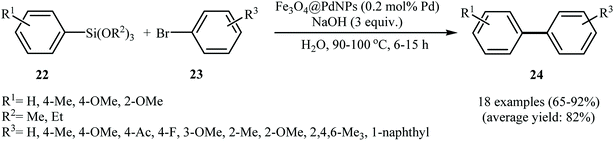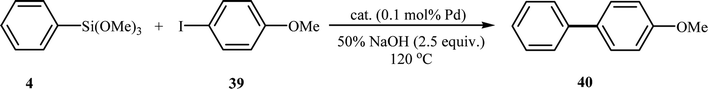Open Access Article
Open Access ArticleRecent advances in the application of nano-catalysts for Hiyama cross-coupling reactions
Aazam Monfared
a,
Robab Mohammadi
a,
Sheida Ahmadi
a,
Mohammad Nikpassand
 *b and
Akram Hosseinian
*b and
Akram Hosseinian
 c
c
aDepartment of Chemistry, Payame Noor University, P. O. Box, 19395-3697, Tehran, Iran
bDepartment of Chemistry, Rasht Branch, Islamic Azad University, Rasht, Iran. E-mail: nikpassand@iaurasht.ac.ir
cSchool of Engineering Science, College of Engineering, University of Tehran, P. O. Box 11365-4563, Tehran, Iran
First published on 23rd January 2019
Abstract
This mini-review highlights the recent developments in the field of metal nanoparticle (NP) catalyzed Hiyama cross-coupling reactions. Most of the nanocatalysts outlined here allow convenient and green synthetic pathways for the construction of carbon–carbon bonds in water and fluoride-free conditions. Literature has been surveyed from 2005 to February 2018.
1. Introduction
Transition-metal-catalyzed cross-coupling reactions have become a routine, straightforward, and powerful method for the preparation of carbon–carbon1 and carbon–heteroatom2 bonds in synthetic organic chemistry. Among the various carbon–carbon cross-coupling reactions, Heck, Stille, Negishi, Sonogashira, Suzuki, Hiyama, and Kumada–Corriu reactions find maximum application in industrial processes.3 In comparison with Suzuki coupling with its problems in preparing and purifying arylboronic acids and the Negishi, Stille, and Kumada–Corriu coupling reactions that use unstable organozinc, toxic organotin, and violent organomagnesium reagents, respectively, the Hiyama coupling uses inexpensive, low-toxicity, broadly available, and chemically stable organosilane reagents making these coupling reactions more attractive from an environmental point of view.4,5 However, the less polarized carbon–silicon bonds of organosilicon reagents make these reagents less reactive toward electrophiles than other organometallic nucleophiles.4 This weak bond requires fluoride ions for its activation and this can be considered as the main disadvantage for this coupling reaction.6Transition-metal nanoparticles (NPs) have attracted extraordinary attention in recent years for potential applications in catalysts due to their high surface to volume ratio and reactive morphologies.7–9 Without the slightest doubt, their use in cross-coupling reactions constitutes one of their most important applications.10–12 In the last decade, the use of metal nanoparticles as highly effective catalysts in Hiyama cross-coupling reactions has attracted more and more attention. Interestingly, in the presence of these catalysts, numerous fluoride-free Hiyama coupling reactions have been reported and the interest in this environmentally friendly reaction has increased. In continuation of our recent works,8,13,14 in this review, we will highlight the advances in metal nanoparticle catalyzed Hiyama coupling reactions from 2005 to February 2018, hoping that it will stimulate researchers to develop truly efficient catalytic systems for this interesting and important coupling reaction.14 It is noted that we have classified these reactions based on the starting materials (e.g. coupling of aryl siloxanes with aryl halides and coupling of vinyl silanes with aryl halides) and the type of catalysts (monometallic nanoparticles and bimetallic nanoparticles).
2. Coupling of aryl siloxanes with aryl halides
The biaryl moiety is the core structure of several drugs such as losartan (anti-hypertensive), telmisartan (anti-hypertensive), knipholone (anti-plasmodial), felbinac (anti-inflammatory), imatinib (anti-cancer), and febuxostat (gout and hyperuricemia treatment). It is also present in several pesticides.15 Numerous synthetic methods have been designed for the preparation of functionalized biaryls with the majority of them relying on the carbon–carbon cross-coupling reactions.15,16 One of the most common processes involves the metal-catalyzed Hiyama coupling reactions of aryl siloxanes with aryl halides. Over the past decade, several mono- and bi-metallic nanoparticles have been developed as effective catalysts for these environmentally friendly reactions. In this section, we highlight the advances in this novel and interesting research arena. The section is divided into two major subsections. The first will discuss monometallic nanoparticles catalyzed coupling reactions, while the second focuses exclusively on bimetallic nanoparticles catalyzed reactions.2.1. Monometallic nanocatalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (particle size of 9.70 nm).
1 ratio (particle size of 9.70 nm).
 | ||
| Scheme 1 Hiyama coupling of aryl siloxanes 1 with aryl bromides 2 catalyzed by a Pd NP in water developed by Sarkar. | ||
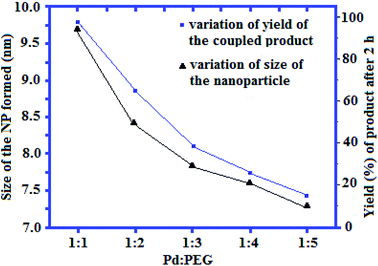 | ||
| Fig. 1 Plot of the size of NP as a function of Pd/PEG molar ratio and yield (%) of the coupled product. | ||
Subsequently, in a closely related investigation, the group of Ranu also described that the cross-coupling of various aryl siloxanes with aryl iodides/bromides in the presence of in situ generated palladium nanoparticles (3–6 nm) from Na2PdCl4/sodium dodecyl sulfate produced the corresponding substituted biaryls in good to excellent yields. Noteworthy, the catalyst was reusable and could be recovered and reused for three reaction runs with only a gradual loss of efficiency. Mechanistically, the reaction is believed to proceed through the standard oxidative addition/transmetallation/reductive elimination sequential process (Scheme 2).18
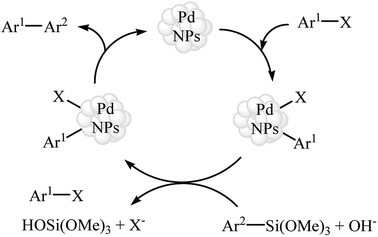 | ||
| Scheme 2 Plausible mechanism for the Pd NPs-catalyzed coupling of aryl siloxanes 1 with aryl bromides 2 in water. | ||
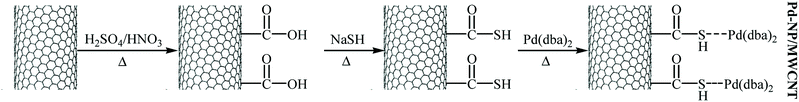
In 2010, Komáromi, Szabó, and Novák compared the catalytic activity of several commercially available Pd/charcoal catalysts in Hiyama cross-coupling reaction of trimethoxyphenylsilane 4 with 3-bromotoluene 5 employing PPh3 as a ligand and tetrabutylammonium fluoride (TBAF) as an activating additive in DMF (Fig. 2). Investigations showed that some catalysts had completely different activity in this reaction. The Pd/C catalyst supplied by Merck and the palladium catalyst deposited onto the multi-walled carbon nanotubes (MWCNT) gave almost full conversions, but Norit A and Evonik 196 KP/D did not show significant catalytic activity. This results showed the importance of the choice of carbon carrier. It is noted that the choice of DMF and TBAF was also crucial for the reaction; other solvents and fluoride sources proved to be unsuitable for this coupling.19 Inspired by this work, Kim and co-workers designed a novel multiwall carbon nanotube supported palladium nanoparticles (Pd-NP/MWCNT) based on the anchoring of Pd-NPs onto the surface of thiolated carbon nanotube, as shown in Fig. 3. The authors explained that Pd-NPs are anchored to the surface of the CNTs due to their interaction with the free electron pairs of the S atoms. The catalytic activity of the system was investigated for Hiyama coupling reaction of trimethoxyphenylsilane and 4-iodotoluene in the presence of TBAF as an activator in p-xylene at 50 °C. The expected 4-methylbiphenyl was obtained in 98% yield.20
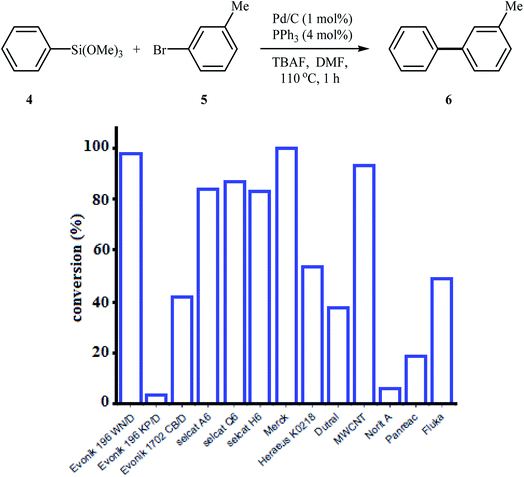 | ||
| Fig. 2 Comparison of the catalytic activity of several commercially available Pd/charcoal catalysts in Hiyama cross-coupling reaction of trimethoxyphenylsilane 4 with 3-bromotoluene 5. | ||
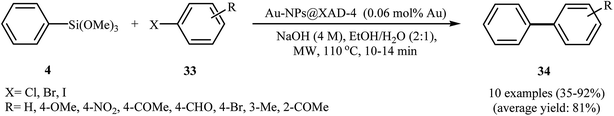
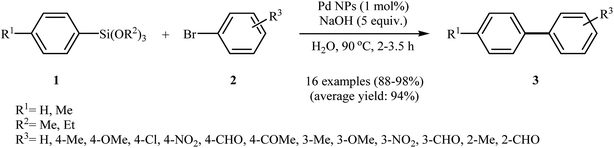
In an innovative design, Shah and Kaur impregnated Amberlite XAD-4, a non-functional macroporous commercial resin, with palladium nanoparticles to obtain a Pd-NPs@XAD-4 catalyst. The method used for nanoparticles impregnation was simple and involved sorption of Pd(OAc)2 in the resin followed by reduction using aqueous NaBH4 solution. Transmission electron microscopy (TEM) images demonstrated that these conditions furnished palladium nanoparticles with size ranging from 5 to 10 nm. The catalytic utility of the catalyst was investigated for Hiyama cross-coupling reactions. Thus, a variety of functionalized biaryls 8 were synthesized via the Pd-NPs@XAD-4 catalyzed coupling of trimethoxyphenylsilane 4 with aryl bromides/chlorides 7 under microwave irradiation and basic conditions (Scheme 3). The protocol is also applicable for the coupling of heteroaryl halides with aryl siloxanes. Broad substrate scope, high turnover number (TON), turnover frequency (TOF) and yields were the merits of this synthetic protocol. Notably, the catalyst could be recovered and reused for five reaction runs with no remarkable loss of activity.21 In a related study, Becht and Drian along with their co-workers prepared a phosphine-derived polystyrene-supported palladium catalyst and successfully applied it for the Hiyama cross-coupling reactions of aryl siloxanes with aryl iodides in toluene at 100 °C. A broad spectrum of substituted aryl iodides were reacted with different aryl siloxanes in the presence of only 0.1 mol% of supported palladium, furnishing the corresponding biaryls in good to excellent yields.22
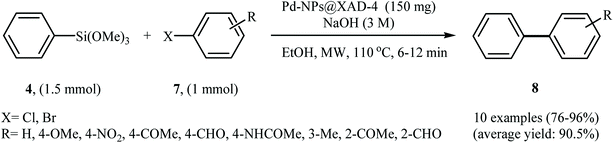 | ||
| Scheme 3 Pd-NPs@XAD-4 catalyzed coupling of trimethoxyphenylsilane 4 with aryl bromides/chlorides 7 reported by Shah and Kaur. | ||
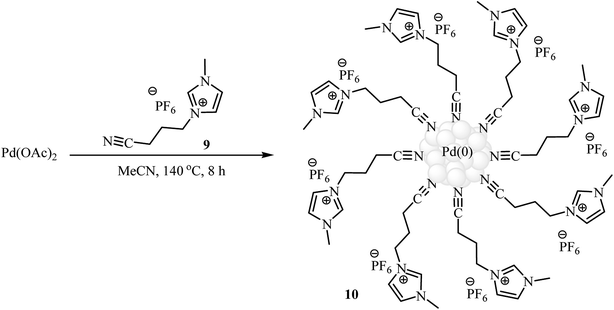
In 2013, Premi and Jain developed an efficient Pd NPs-containing ionic liquid 10 {Pd[CN-bmim]PF6} by simple heating (140 °C) of Pd(OAc)2 with nitrile-functionalized 3-(3-cyanopropyl)-1-methyl-1H-imidazol-3-ium hexafluorophosphate 9 {[CN-bmim]PF6} in acetonitrile (Fig. 4). The size of the Pd nanoparticles was determined by DLS, TEM, and SEM and revealed the catalyst presented an average particle size range of 2–5 nm. This ionic liquid was tested as an in situ catalyst in the Hiyama cross-coupling of trimethoxyphenylsilane 4 with (hetero)aryl halides 12 in the presence of 1-butyl-3-methylimidazolium fluoride 11 {[bmim]F}as an activator, providing good yields and selectivities of coupling products 13 (Scheme 4a). The catalyst was reused four times maintaining the high yields. The proposed mechanism for this reaction involves the initial formation of aryl–Pd complex A through the oxidative addition of (hetero)aryl halide 12 to the Pd[CN-bmim]PF6 catalyst. This intermediate A upon anion exchange with [bmim]F leads to the intermediate B which undergoes transmetalation with trimethoxyphenylsilane 4 to afford intermediate complex C. Finally, reductive elimination of this complex produces the observed biaryls 13 (Scheme 4b).23
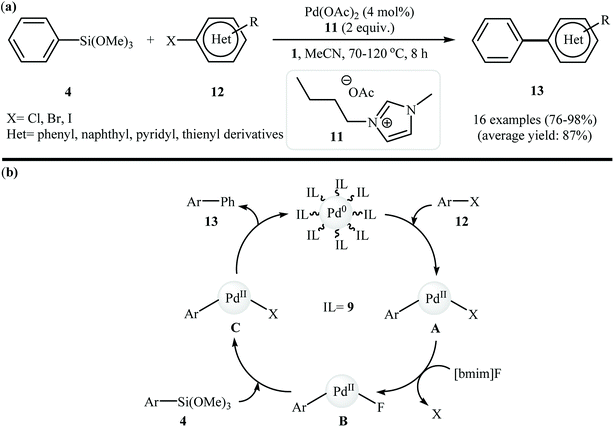 | ||
| Scheme 4 (a) Jain's synthesis of biaryls 13; (b) mechanistic proposal for the formation of biaryls 13. | ||
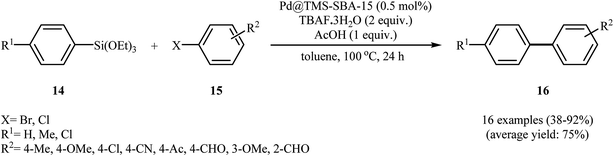
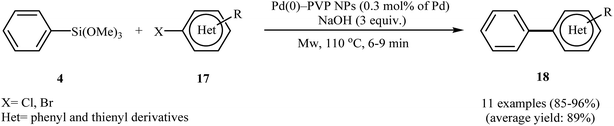
In 2014, the trimethylsilylated mesoporous SBA-15 (TMS-SBA-15) was used for immobilizing Pd(0) nanoparticles, by Huang et al.24 The catalytic activity of the obtained catalyst, Pd@TMS-SBA-15 was investigated for the synthesis of biaryls 16 via the Hiyama cross-coupling reaction of triethoxyphenylsilanes 14 with aryl bromides/chlorides 15 in the presence of tetra-n-butylammonium fluoride trihydrate (TBAF·3H2O) as a fluoride source and AcOH as a proton source at 100 °C. The results established that the low amount of the prepared catalyst (0.5 mol%) could furnish the expected coupling products in moderate to high yields (Scheme 5). However, 4-chloroanisole failed to participate in this reaction and sterically hindered 2-bromoanisole gave the corresponding product in only 13% yield. Notably, an attempt of catalyst reuse showed noticeable loss of catalytic activity from 92% in the first run to approximately 22% in the fourth run. The authors compared the catalytic activity of the catalyst with Pd@P-SBA-15, Pd@PS-S15, and Pd/C. The results demonstrated superior catalytic activity of the synthesized catalyst (Table 1). In the same year, Shah and Kaur found that ligand-free cross-coupling of trimethoxyphenylsilane 4 with aryl halides 17 could be successfully carried out using polyvinylpyrrolidone (PVP) stabilized colloidal palladium nanoparticles (2–5 nm) [Pd(0)–PVP NPs] as the catalytic system and sodium hydroxide as the activator under microwave irradiation (Scheme 6). Noteworthy, the catalyst could be reused for four reaction runs without significant loss of activity.25
With the objective of designing a greener procedure to biaryls through palladium catalyzed Hiyama coupling reactions, Ohtaka and his group were able to demonstrate that linear polystyrene-stabilized PdO nanoparticles (PS-PdONPs) could efficiently catalyze the coupling of aryl trimethoxysilanes 19 with aryl bromides 20 in water under fluoride-free conditions. The process was carried out in the presence of 1.0 equiv. of tetrabutylammonium chloride (TBAC) under basic conditions, and provided functionalized biaryls 21 in moderate to high yields (Scheme 7). However, sterically hindered ortho-substituted aryl halides did not work well under this conditions. The catalyst was reusable and could catalyze five reaction cycles without detrimental loss of catalytic activity. The TEM image of the recovered catalyst showed that the size of the nanoparticles (2.1 ± 0.3 nm) was maintained even after the third run. Moreover, leaching of palladium species was not detected by ICP-AES analysis. The same reaction was also performed by using PS-PdNPs in place of PS-PdONPs. Surprisingly, under this reaction conditions the expected coupling product was not observed. The authors suggested that the difference in catalytic activity between PS-PdONPs and PS-PdNPs may be caused by the difference in mechanisms.26
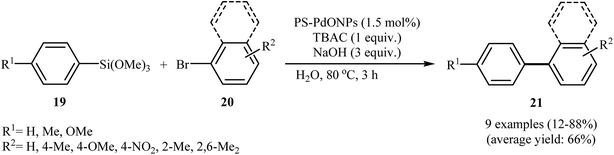 | ||
| Scheme 7 Carbon–carbon cross-coupling reaction between aryl trimethoxysilanes 19 with aryl bromides 20 using Ps-PDONPs as catalyst in water. | ||
Very recently, the same research team studied a detailed mechanism for the Hiyama coupling reaction in water using PS-PdONPs as a catalyst. Stepwise reactions, hot filtration, and leaching tests showed that this reaction proceeds through a different mechanism from the commonly accepted one that starts from the oxidative addition of an aryl halide to a Pd(0) species. As shown in Scheme 8, the proposed Pd(II) catalytic cycle starts with a local leaching of palladium under basic condition. Next, this palladium species reacts with aryl trimethoxysilane and then immediately re-stabilizes on the catalyst surface. Finally, the generated Pd–Ar species reacts with aryl bromide to give the expected product.27
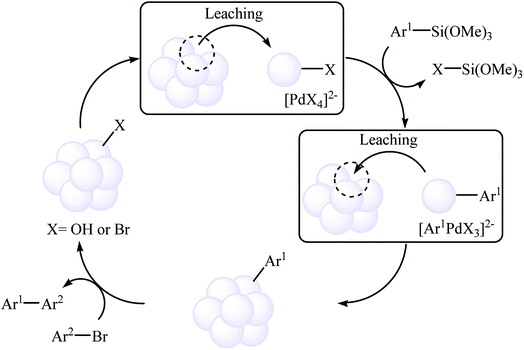 | ||
| Scheme 8 Mechanistic proposal for the reaction in Scheme 7. | ||
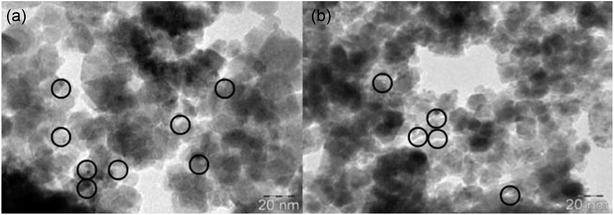
Recently, a range of magnetic nanoparticles (MNPs) have been developed and exploited as catalysts for a variety of chemical transformations. Among the various magnetic nanoparticles, Fe3O4 nanoparticles have drawn much attention because these chemically stable nanoparticles can be prepared by simple methods, which can be used as an efficient alternative to heterogeneous catalyst supports.28 In 2011, Sreedhar, Kumar, and Yada reported magnetically separable Fe3O4@PdNPs consisting of Fe3O4 NPs of 25–50 nm in diameter and PdNPs of 5 nm encaged in Fe3O4 as highly active catalyst for fluoride-free Hiyama cross-coupling reaction (Scheme 9). The reported catalyst was highly active for differently substituted aryl siloxanes 22 and aryl bromides 23, and resulted in a high yield of corresponding biaryl 24 using NaOH as a base in water. After the reaction catalyst was recovered by an external magnet and reused for five runs with only a small decrease in the yield. Fig. 5 shows TEM images of Fe3O4@PdNPs and clearly confirms that no change in the morphology took place even after five cycles.29 In a closely related investigation, Lee and co-workers also synthesized a series of biaryls through the reaction of corresponding aryl halides and phenyltrimethoxysilane using Fe3O4@PdNPs/KF/TBAI/DMA as coupling system.30
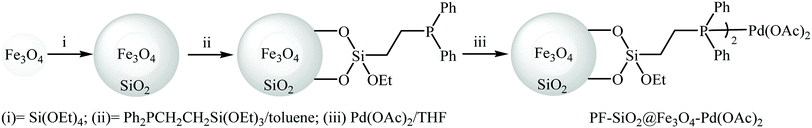
Phosphine-functionalized SiO2@Fe3O4 was employed by Zhang and co-workers for immobilizing Pd nanoparticles. The synthetic approach included preparation of Fe3O4-NPs (20 nm) coated silica (SiO2@Fe3O4) through a sol–gel approach followed by reaction with 2-(diphenylphosphino)ethyltriethoxysilane to afford PF-SiO2@Fe3O4. The PF-SiO2@Fe3O4–Pd(OAc)2 nanoparticles were generated upon treatment of PF-SiO2@Fe3O4 with Pd(OAc)2 in THF (Fig. 6). The PF-SiO2@Fe3O4–Pd(OAc)2 was employed as an efficient and reusable catalyst for Hiyama reaction of phenyltrimethoxysilane 4 with aryl halides 25 in THF. Various aryl iodides and bromides with different electron densities could be used successfully in this protocol to afford the corresponding biaryls 26 in high to excellent yields (Scheme 10). However, no cross-coupling product was detected when phenyltrimethylsilane was used as a coupling partner under this reaction conditions. It is noted that the catalyst could be simply recovered and reused for 10 times with no detectable deactivation.31
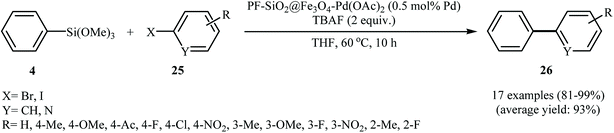 | ||
| Scheme 10 C–C cross-coupling of phenyltrimethoxysilane 4 with aryl halides 25 catalyzed by PF-SiO2@Fe3O4–Pd(OAc)2. | ||
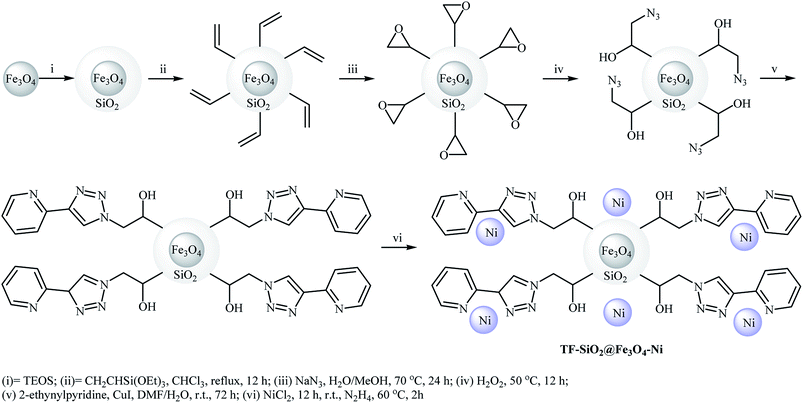
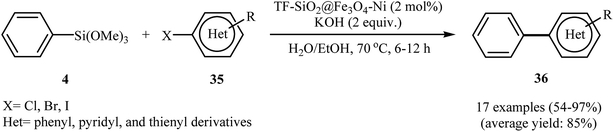
Very recently, the group of Karami employed Fe3O4@SiO2/APTMS (APTMS = 3-aminopropyltrimethoxysilane) core–shell nanocatalyst support for stabilization of Pd(cdha)2 (cdha = bis(2-chloro-3,4-dihydroxyacetophenone)) and developing a novel catalyst, Fe3O4@SiO2/APTMS/Pd(cdha)2 (Fig. 7). The authors fully characterized the structure and composition of this catalyst by using various analyses such as TEM, SEM, XRD, and FT-IR. According to the TEM images, the average sizes of Fe3O4 in Fe3O4@SiO2/APTMS and palladium in Fe3O4@SiO2/APTMS/Pd(cdha)2 nanoparticles are about 23.8 nm and 15.8 nm respectively. The utility of the catalyst, was studied for fluoride-free Hiyama cross-coupling reactions of aryl halides with phenyltrimethoxysilane. It was found that the solvent, activator, and reaction temperature could affect the process. The best results were obtained in the presence of NaOH/sodium dodecylsulfate (SDS) combination as the activating system at 100 °C and water as the solvent. It should be mentioned that the catalyst was easily separated from the reaction mixture by the use of an external magnetic field and was efficiently reused for four cycles keeping almost the same activity in each cycle.32
Zinc oxide nanoparticles have recently emerged as promising supports for immobilization of metal nanoparticles. ZnO-supported palladium nanoparticles have been extensively studied for cross-coupling reactions.33 In 2015, Sarvari and Razmi synthesized ZnO-supported Pd nanoparticles through the chemical precipitation (CP) method. The method involves the reaction of Pd(NO3)2 and Zn(NO3)2 in aqueous solution of Na2CO3 (1 M) for 2 h and then filtration of the resulted precipitate. The catalytic activity of heterogeneous nano Pd/ZnO catalyst was studied for Hiyama coupling reaction between phenyl trimethoxysilane 4 and aryl halides 27 in the presence of K2CO3 as a base in ethyleneglycol. The corresponding biaryls 28 were obtained in high to excellent yields (Scheme 11). The catalytic system was also efficient in the Suzuki–Miyaura cross-coupling reaction. Furthermore, it could be reused ten times without obvious decrease of the catalytic activity.34
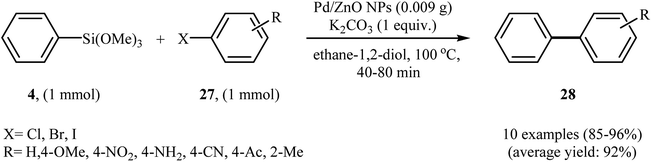 | ||
| Scheme 11 Synthesis of functionalized biaryls 28 through Pd/ZnO NPs-catalyzed reaction of phenyl trimethoxysilane 4 and aryl halides 27 in ethyleneglycol. | ||
Very recently, in a related investigation, the same research team described that the Hiyama coupling reaction of phenyl trimethoxysilane with aryl halides in the presence of Pd/ZnO NPs/Cs2CO3 combination as a catalytic system under visible-light irradiation at room temperature produced the desired biaryls in good yields.35
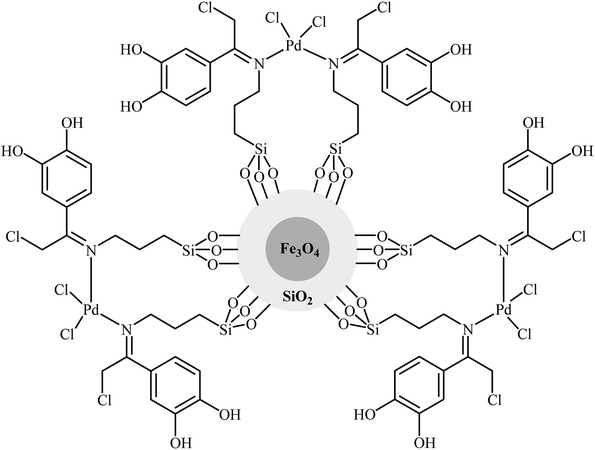
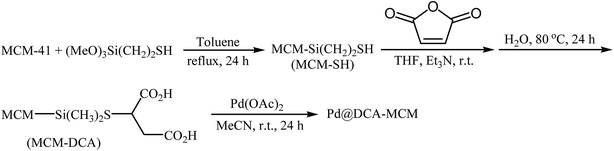
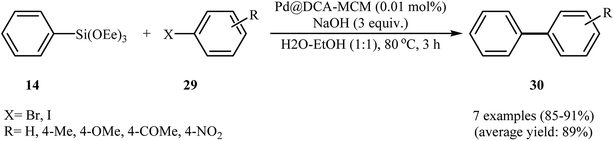
In 2018, Bazgir and his team developed a novel heterogeneous catalyst (Pd@DCA-MCM; DCA-MCM = 1,2-dicarboxylic acid-functionalized MCM-41) via a four-step procedure through the reaction of MCM-41 with 2-(trimethoxylsilyl)ethane-1-thiol and subsequent reaction of resulted MCM-SH with maleic anhydride followed by hydrolysis at 80 °C and finally reaction of prepared MCM–DCA with an acetonitrile solution of palladium acetate at room temperature (Scheme 12).36 The catalyst has been characterized by FT-IR, TGA, XRD, AAS, TEM, SEM, and EDS measurements. The size of the palladium nanoparticles was found to be 10–12 nm by TEM technique. The Pd@DCA-MCM nanocomposite exhibited a high catalytic activity in the Hiyama cross-coupling reaction of triethoxy(phenyl)silane 14 and aryl halides 29 in H2O–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under fluoride-free conditions. The results showed that the corresponding biaryls 30 were readily formed in excellent yields after 3 h (Scheme 13). The catalyst was also successfully employed for Suzuki and Sonogashira cross-coupling reactions. The reusability of the catalyst was investigated in the Suzuki reaction of phenylboronic acid and phenyl iodide. The catalyst could be reused five times without observable loss of activity.
1) under fluoride-free conditions. The results showed that the corresponding biaryls 30 were readily formed in excellent yields after 3 h (Scheme 13). The catalyst was also successfully employed for Suzuki and Sonogashira cross-coupling reactions. The reusability of the catalyst was investigated in the Suzuki reaction of phenylboronic acid and phenyl iodide. The catalyst could be reused five times without observable loss of activity.
Most recently, Kandathil and co-workers reported the synthesis of palladium nanoparticles through a green approach by using an aqueous-ethanolic extract of black pepper (Piper nigrum) (Fig. 8).37 The formation of Pd NPs was confirmed by various analytical techniques like XRD, FE-SEM, EDS, TEM, ATR-IR, UV–Vis spectroscopy, BET, TGA and ICP-OES analysis. The attenuated total reflectance infrared spectroscopy (ATR-IR) revealed that functional groups like –OH, –NH, aromatic and aliphatic –CH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C
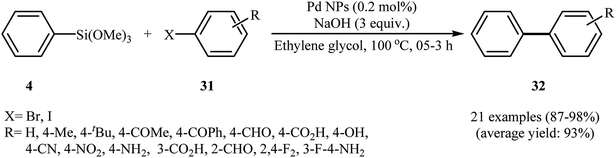
O and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the biomolecules (e.g., phenols, acids, pellitorine, ethyl piperonyl cyanoacetate, piperine and N-isobutyl-tetradeca-2,4-dienamide) had the most affinity for binding to Pd(II), and thereby reducing it to Pd(0) and forming of Pd NPs. The biosynthesized Pd NPs were successfully employed as catalysts for the synthesis of a wide range of biaryls 32 via Hiyama cross-coupling of phenyl trimethoxysilane 4 and aryl halides 31 in the presence of NaOH in ethylene glycol at 100 °C. This protocol furnished the functionalized biaryls with use of a very low amount of catalyst (0.2 mol%) in excellent to almost quantitative yields (Scheme 14) with no need to any fluoride source. Interestingly, the Pd NPs could be easily from the final reaction mixture by centrifugation (6000 rpm for 15–30 min), and then be reused up to ten times without considerable loss in the catalytic activity.
C in the biomolecules (e.g., phenols, acids, pellitorine, ethyl piperonyl cyanoacetate, piperine and N-isobutyl-tetradeca-2,4-dienamide) had the most affinity for binding to Pd(II), and thereby reducing it to Pd(0) and forming of Pd NPs. The biosynthesized Pd NPs were successfully employed as catalysts for the synthesis of a wide range of biaryls 32 via Hiyama cross-coupling of phenyl trimethoxysilane 4 and aryl halides 31 in the presence of NaOH in ethylene glycol at 100 °C. This protocol furnished the functionalized biaryls with use of a very low amount of catalyst (0.2 mol%) in excellent to almost quantitative yields (Scheme 14) with no need to any fluoride source. Interestingly, the Pd NPs could be easily from the final reaction mixture by centrifugation (6000 rpm for 15–30 min), and then be reused up to ten times without considerable loss in the catalytic activity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 16). The catalyst could be recovered by an external magnet, and further reused for more than six consecutive times without significant loss of activity.38
1 (Scheme 16). The catalyst could be recovered by an external magnet, and further reused for more than six consecutive times without significant loss of activity.38
2.2. Bimetalic nanoparticles
Recently, bimetallic nanoparticle catalysts have drawn intense attention because of their great catalytic performance39 and low production costs.40 Accordingly, a huge variety of bimetallic nanoparticles have been prepared and applied as catalysts for carbon–carbon cross-coupling reactions.41 In this section, we will give a general overview of the recent advances in bimetallic nanoparticle catalyzed Hiyama cross-coupling reactions.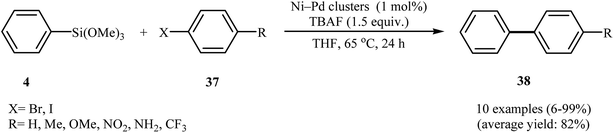 | ||
| Scheme 17 Palladium-coated nickel nanoclusters catalyzed synthesis of biaryls 38 reported by Pachón. | ||
A decade later, the group of Nájera investigated bimetallic palladium–nickel nanoparticle catalyst supported on multiwall carbon nanotubes with varying Pd to Ni atomic ratios for Hiyama coupling of phenyl trimethoxysilane 4 with 4-iodoanisole 39 in the absence of a fluoride source in water (Table 2). An almost quantitative yield of expected biaryl 40 was obtained with Pd70Ni30/MWCNTs. Notably, the same results were also obtained by using monometallic Pd NPs supported in MWCNTs as the catalyst. The bimetallic Ni–Pd nanoparticle catalysts were also examined for the Hiyama, Heck and Sonogashira reactions. Excellent yields were obtained for these coupling reactions using Ni50Pd50/MWCNTs catalyst at 120 °C in water.43
3. Coupling of vinylsilanes with aryl halides
In 2011, Chatterjee, Dey, and Ranu have described the synthesis of functionalized styrenes 43 through the Hiyama cross-coupling of triethoxyvinylsilane 41 with corresponding aryl halides 42 catalyzed by Pd(0) nanoparticles (2–3 nm) generated in situ in the reaction mixture in presence of PdCl2 and TBAF in refluxing THF (65 °C). The author suggested that TBAF playing a dual role in this reaction; the fluoride supplier and the nanoparticle stabilizer. Under the optimized conditions, the reaction tolerated a range of base-sensitive functional groups (COOR, CONHPh, NHCOPh, CN) and provided the coupling products in good to high yields (Scheme 18). Noteworthy, styrenyl-, cinnamyl- and dienyl-halides have also been employed as viable electrophilic partners in this cross-coupling reaction and provided corresponding trans aryl 1,3-, 1,4- and 1,5-dienes in high yields and excellent stereoselectivity. It should be mentioned that the attempts to recycle the catalyst were not successful as after the first cycle the nanoparticles grew bigger (10–15 nm), leading to lower yields and being less efficient.45 Later, Grirrane and co-workers compared the catalytic activity of several solid supported palladium nanoparticles (Pd/MgO, Pd/TiO2, Pd/CeO2 and Pd/C) for the Hiyama C–C coupling reaction of a range of aryl iodides and vinylsilanes using ligand-free conditions in DMF. The results showed that the most active catalysts for this coupling reaction were those in which palladium is supported on MgO and TiO2. Interestingly, the analogous Pt and Au materials were inefficient to promote this reaction.46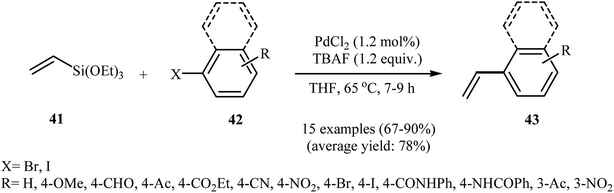 | ||
| Scheme 18 Synthesis of styrenes 43 via PdNPs-catalyzed coupling of triethoxyvinylsilane 41 with corresponding aryl halides 42. | ||
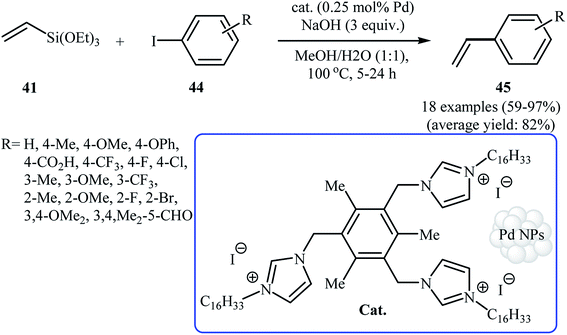
In 2014, Planellas and co-workers prepared palladium NPs by the hydrogenation of Pd(dba)2 in the presence of a trisimidazolium iodide as the stabilizer, and studied their catalytic activity in Hiyama coupling reaction of triethoxyvinylsilane 41 with aryl iodides 44 in binary solvent toluene/EtOH with ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 19). Under optimized conditions [Pd-NPs (0.25 mol% Pd), NaOH (3 equiv.), H2O/MeOH (1
1 (Scheme 19). Under optimized conditions [Pd-NPs (0.25 mol% Pd), NaOH (3 equiv.), H2O/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 100 °C], the desired biaryls 45 were obtained in moderate to almost quantitative yields. The catalyst was also highly active for Heck and copper-free Sonogashira reactions.47
1), 100 °C], the desired biaryls 45 were obtained in moderate to almost quantitative yields. The catalyst was also highly active for Heck and copper-free Sonogashira reactions.47
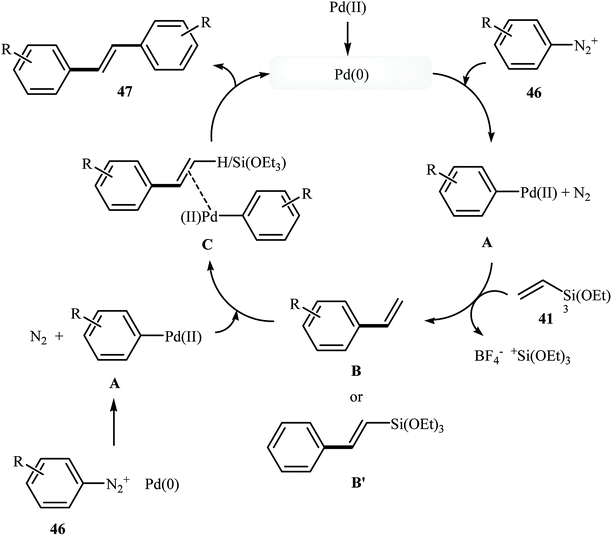
Very recently, Gaikwad's group demonstrated an elegant and innovative Pd NPs-catalyzed sequential Hiyama–Heck reaction of triethoxyvinylsilane 41 with aromatic diazonium salts 46 into symmetrical trans-stilbene derivatives 47 (Scheme 20). Palladium nanoparticles have been generated in situ from Pd(OAc)2 and Triton X-100 in water. The TEM image of the in situ generated catalyst presented a spherical morphology of the nanoparticles with a size below 20 nm. The results showed that the electronic nature and substitution of the diazonium salt did not affect the reaction outcome as yields of the stilbene products obtained a range from 80–95% for the 10 reactions highlighted. After the reaction, aqueous system containing the surfactant along with the Pd NPs was recovered and reused for five runs without any significant loss of catalytic activity. The author proposed reaction mechanism is depicted in Scheme 21.48
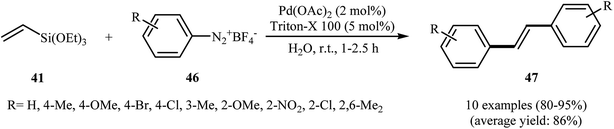 | ||
| Scheme 20 Synthesis of symmetrical trans-stilbenes 47 via Pd NPs-catalyzed sequential Hiyama–Heck reaction of triethoxyvinylsilane 41 with aromatic diazonium salts 46. | ||
4. Miscellaneous reactions
In 2008, the Ranu group reported an elegant in situ-generated palladium(0) nanoparticles catalyzed cross-coupling reaction of aryl and vinyl siloxanes 48 with allyl acetates 49 into stereoselective (E)-coupling products 50 employing PdCl2/tetrabutyl ammonium bromide (TBAB) as a catalytic system in THF (Scheme 22). The reactions were generally highly stereoselective, and (E)-coupling products were obtained both from cis and trans allyl acetates. Notably, the Pd(0) nanoparticles could be recovered and then reused up to three times with only a small loss of catalytic activity. However, the catalyst could not perform well for fourth run and a steep loss in the product yield of approximately 60% (after 3rd run) and 20% (after 4th run) was observed.49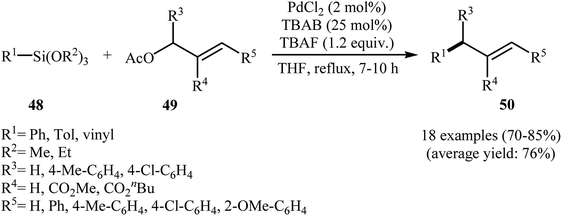 | ||
| Scheme 22 PdNPs-catalyzed cross-coupling reaction of aryl and vinyl siloxanes 48 with allyl acetates 49 developed by Ranu. | ||
Following this work, Sarkar and his group were able to demonstrate that in situ generated Pd nanoparticles (by heating 0.04 mmol of K2PdCl4 and 1.16 mmol of PEG-600 at 70 °C for 15 min) could efficiency catalyze Hiyama coupling reaction of aryl siloxanes 51 with benzyl halides 52 to the corresponding diarylmethanes 53 in high to excellent yields (Scheme 23). The reaction showed excellent functional group tolerance and could be applied for the synthesis of a diverse range of functionalized diarylmethanes with potential biological activities.50
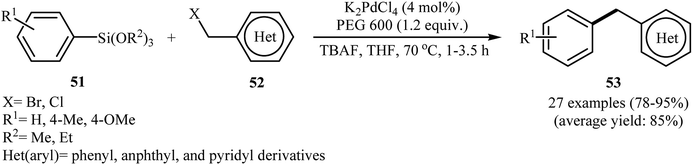 | ||
| Scheme 23 Synthesis of diarylmethanes 53 via PdNPs-catalyzed Hiyama coupling of aryl siloxanes 51 with benzyl halides 52. | ||
Recently, Song and co-workers developed an in situ-generated Pd-NPs (2.69 ± 0.83 nm) catalyzed three-component coupling reaction of trimethoxyallylsilane 54, chloromethyl(heter)oarenes 55, and carbon dioxide, which allowed for the synthesis of α,β-unsaturated esters 56 in good to high yields (Scheme 24). This carboxylative coupling reaction took place under mild reaction conditions (1.0 MPa pressure of CO2 and 50 °C temperature) in the presence of 5 mol% of Pd(acac)2 as a precatalyst and 2 equiv. of TBAF as an additive in DMF and tolerated considerable functionality. The additive (TBAF) played key dual roles in this reaction; the stabilizer and the activator. According to the author proposed mechanism, the reaction proceeded via an oxidative addition/transmetalation/coordination/nucleophilic addition/reductive elimination/isomerization sequential process.51
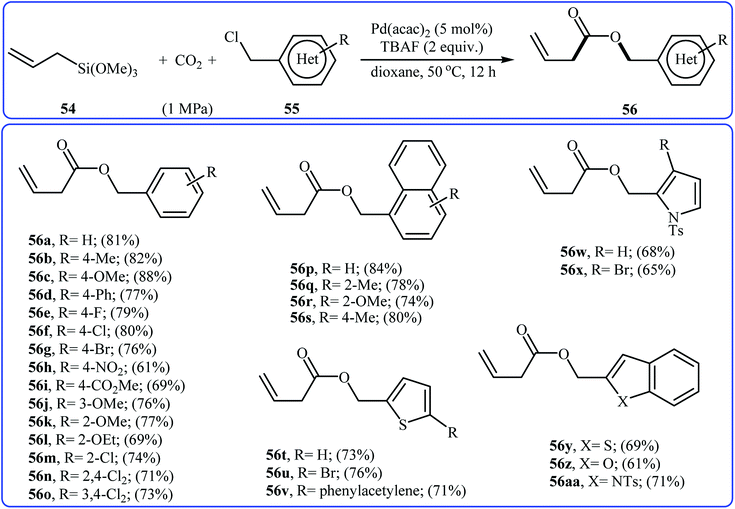 | ||
| Scheme 24 PdNPs-catalyzed carboxylative coupling of trimethoxyallylsilane 54 with chloromethyl(heter)oarenes 55 reported by Song et al. | ||
5. Conclusion
Transition-metal-catalyzed carbon–carbon cross-coupling reactions have emerged as the most efficient methodologies for the syntheses of many molecules ranging from pharmaceuticals to material science to optical devices. Among the various carbon–carbon cross-coupling reactions, Heck, Stille, Negishi, Sonogashira, Suzuki, Hiyama, and Kumada–Corriu reactions find maximum application in industrial processes. Undoubtedly, Hiyama cross-coupling of low-cost, non-toxic, and easily available organosilane reagents in comparison to the Suzuki, Negishi, Stille, and Kumada–Corriu coupling reactions is an environment-friendly protocol for the formation of the new C–C bonds. However, silicon-based cross-coupling reactions often require heating in the presence of a fluoride source (e.g. CsF, KF, AgF, TBAF, TBAT, and TASF) which has significantly hampered their widespread acceptance. To address the “fluoride problem”, recently, several metal-based nanocatalysts have been developed that due to their very high surface-to-volume ratio compared to bulk catalytic materials and reactive morphologies allow for fluoride-free Hiyama coupling under relatively mild conditions, with the benefits of high product yield, low catalyst loading, and ease of catalyst separation. As illustrated, most of the nanoparticles catalyzed Hiyama coupling reactions covered in this review could be easily achieved in the most environmentally benign solvent, water. These results clearly show the potential application of these reactions in industry. Despite the significant and revolutionary achievements during the past few years in this research arena, many challenges still remain to be overcome: (a) the number of reported examples in some reactions such as coupling of vinylsilanes with aryl halides/aromatic diazonium salts and coupling of aryl siloxanes with benzyl/alkyl halides are narrow and there is further need to study the scope and limitations of these reactions; (b) most of the nanoparticle catalyzed Hiyama reactions are limited to the use of monometallic catalysts. Thus the exploration of bi-metallic, tri-metallic, and multi-metallic nanoparticles (BNPs, TNPs, and MNPs) are highly desirable in term of the cost of preparation of the catalysts; and (c) the coupling of readily available and low-cost aryl chlorides remained challenging and more difficult than the corresponding aryl bromides and iodides. Therefore the development of truly efficient catalytic systems which can accomplish the coupling of aryl chlorides and organosilanes with high yields is strongly needed. It is our hope that this review will stimulate researchers to further research and thinking in this interesting and important research field.Conflicts of interest
There are no conflicts to declare.References
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (b) H. Doucet and J. C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834–871 CrossRef CAS PubMed; (c) D. Roy and Y. Uozumi, Adv. Synth. Catal., 2018, 360, 602–625 CrossRef CAS.
- (a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596–1636 CrossRef CAS PubMed; (b) F. M. Tappe, V. T. Trepohl and M. Oestreich, Synthesis, 2010, 3037–3062 CAS; (c) M. Abdoli, Z. Mirjafary, H. Saeidian and A. Kakanejadifard, RSC Adv., 2015, 5, 44371–44389 RSC.
- F. Diedrich and A. De Meijere, Metal catalyzed cross-coupling reactions, vol. 1, Wiley-VCH Verlag GmbH, Weinheim, 2004 Search PubMed.
- Y. Nakao and T. Hiyama, Chem. Soc. Rev., 2011, 40, 4893–4901 RSC.
- (a) F. Foubelo, C. Nájera and M. Yus, Chem. Rec., 2016, 16, 2521–2533 CrossRef CAS PubMed; (b) S. Sarhandi, M. Daghagheleh, M. Vali, R. Moghadami and E. Vessally, Chem. Rev. Lett., 2018, 1, 9–15 Search PubMed.
- D. Shah and H. Kaur, Curr. Catal., 2015, 4, 224–230 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743–754 RSC.
- E. Vessally, M. Babazadeh, A. Hosseinian, S. Arshadi and L. Edjlali, J. CO2 Util., 2017, 21, 491–502 CrossRef CAS.
- D. Wang and D. Astruc, Chem. Rev., 2014, 114, 6949–6985 CrossRef CAS PubMed.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC.
- A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973–4985 RSC.
- R. K. Sharma, S. Dutta, S. Sharma, R. Zboril, R. S. Varma and M. B. Gawande, Green Chem., 2016, 18, 3184–3209 RSC.
- (a) K. Didehban, E. Vessally, A. Hosseinian, L. Edjlali and E. S. Khosroshahi, RSC Adv., 2018, 8, 291–301 RSC; (b) S. Shahidi, P. Farajzadeh, P. Ojaghloo, A. Karbakhshzadeh and A. Hosseinian, Chem. Rev. Lett., 2018, 1, 37–44 Search PubMed; (c) S. Mohammadi, M. Musavi, F. Abdollahzadeh, S. Babadoust and A. Hosseinian, Chem. Rev. Lett., 2018, 1, 49–55 Search PubMed.
- (a) E. Vessally, K. Didehban, R. Mohammadi, A. Hosseinian and M. Babazadeh, J. Sulfur Chem., 2018, 39, 332–349 CrossRef CAS; (b) E. Vessally, R. Mohammadi, A. Hosseinian, K. Didehban and L. Edjlali, J. Sulfur Chem., 2018, 39, 443–463 CrossRef CAS; (c) F. A. H. Nasab, L. Z. Fekri, A. Monfared, A. Hosseinian and E. Vessally, RSC Adv., 2018, 8, 18456–18469 RSC; (d) K. Nejati, S. Ahmadi, M. Nikpassand, P. D. K. Nezhad and E. Vessally, RSC Adv., 2018, 8, 19125–19143 RSC; (e) A. Hosseinian, F. A. H. Nasab, S. Ahmadi, Z. Rahmani and E. Vessally, RSC Adv., 2018, 8, 26383–26398 RSC; (f) A. Hosseinian, S. Farshbaf, L. Z. Fekri, M. Nikpassand and E. Vessally, Top. Curr. Chem., 2018, 376, 23 CrossRef PubMed; (g) A. Hosseinian, S. Ahmadi, F. A. H. Nasab, R. Mohammadi and E. Vessally, Top. Curr. Chem., 2018, 376, 39 CrossRef PubMed; (h) A. Hosseinian, F. A. H. Nasab, S. Ahmadi, Z. Rahmani and E. Vessally, RSC Adv., 2018, 8, 26383–26398 RSC; (i) A. Hosseinian, R. Mohammadi, S. Ahmadi, A. Monfared and Z. Rahmani, RSC Adv., 2018, 8, 33828–33844 RSC; (j) S. Sarhandi, M. Daghagheleh, M. Vali, R. Moghadami and E. Vessally, Chem. Rev. Lett., 2018, 1, 9–15 Search PubMed; (k) A. Hosseinian, S. Farshbaf, R. Mohammadi, A. Monfared and E. Vessally, RSC Adv., 2018, 8, 17976–17988 RSC; (l) S. Farshbaf, L. Z. Fekri, M. Nikpassand, R. Mohammadi and E. Vessally, J. CO2 Util., 2018, 25, 194–204 CrossRef CAS; (m) S. Farshbaf, L. Sreerama, T. Khodayari and E. Vessally, Chem. Rev. Lett, 2018, 1, 56–67 Search PubMed; (n) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 71662–71675 RSC; (o) E. Vessally, L. Edjlali, A. Hosseinian, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 49730–49746 RSC; (p) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 99781–99793 RSC; (q) E. Vessally, S. Soleimani-Amiri, A. Hosseinian, L. Edjlali and A. Bekhradnia, RSC Adv., 2017, 7, 7079–7091 RSC.
- J.-A. García-López and M. F. Greaney, Chem. Soc. Rev., 2016, 45, 6766–6798 RSC.
- I. Hussain, J. Capricho and M. A. Yawer, Adv. Synth. Catal., 2016, 358, 3320–3349 CrossRef CAS.
- D. Srimani, S. Sawoo and A. Sarkar, Org. Lett., 2007, 9, 3639–3642 CrossRef CAS PubMed.
- B. C. Ranu, R. Dey and K. Chattopadhyay, Tetrahedron Lett., 2008, 49, 3430–3432 CrossRef CAS.
- A. Komáromi, F. Szabó and Z. Novák, Tetrahedron Lett., 2010, 51, 5411–5414 CrossRef.
- J. Y. Kim, Y. Jo, S.-K. Kook, S. Lee and H. C. Choi, J. Mol. Catal. A: Chem., 2010, 323, 28–32 CrossRef CAS.
- D. Shah and H. Kaur, J. Mol. Catal. A: Chem., 2012, 359, 69–73 CrossRef CAS.
- C. Diebold, A. Derible, J.-M. Becht and C. Le Drian, Tetrahedron, 2013, 69, 264–267 CrossRef CAS.
- C. Premi and N. Jain, Eur. J. Org. Chem., 2013, 5493–5499 CrossRef CAS.
- S.-H. Huang, C.-H. Liu and C.-M. Yang, Green Chem., 2014, 16, 2706–2712 RSC.
- D. Shah and H. Kaur, Curr. Catal., 2014, 3, 39–46 CrossRef CAS.
- A. Ohtaka, T. Kotera, A. Sakon, K. Ueda, G. Hamasaka, Y. Uozumi, T. Shinagawa, O. Shimomura and R. Nomura, Synlett, 2016, 27, 1202–1206 CrossRef CAS.
- A. Sakon, R. Ii, G. Hamasaka, Y. Uozumi, T. Shinagawa, O. Shimomura, R. k. Nomura and A. Ohtaka, Organometallics, 2017, 36, 1618–1622 CrossRef CAS.
- (a) J. Govan and Y. K. Gun'ko, Nanomaterials, 2014, 4, 222–241 CrossRef PubMed; (b) T. Cheng, D. Zhang, H. Li and G. Liu, Green Chem., 2014, 16, 3401–3427 RSC; (c) B. Karimi, F. Mansouri and H. M. Mirzaei, ChemCatChem, 2015, 7, 1736–1789 CrossRef CAS; (d) A. Baeza, G. Guillena and D. J. Ramón, ChemCatChem, 2016, 8, 49–67 CrossRef CAS.
- B. Sreedhar, A. S. Kumar and D. Yada, Synlett, 2011, 1081–1084 CrossRef CAS.
- W. S. Lee, S. Byun, J. Kwon and B. M. Kim, Bull. Korean Chem. Soc., 2016, 37, 1992–1997 CrossRef CAS.
- L. Zhang, P. Li, H. Li and L. Wang, Catal. Sci. Technol., 2012, 2, 1859–1864 RSC.
- K. Karami, N. Jamshidian, M. M. Nikazma, P. Hervés, A. R. Shahreza and A. Karami, Appl. Organomet. Chem., 2018, 32, e3978 CrossRef.
- (a) M. Hosseini-Sarvari, Z. Razmi and M. M. Doroodmand, Appl. Catal., A, 2014, 475, 477–486 CrossRef CAS; (b) T. Chatterjee, R. Dey and B. C. Ranu, J. Org. Chem., 2014, 79, 5875–5879 CrossRef CAS PubMed.
- M. Hosseini-Sarvari and Z. Razmi, Helv. Chim. Acta, 2015, 98, 805–818 CrossRef CAS.
- M. Hosseini-Sarvari and Z. Bazyar, ChemistrySelect, 2018, 3, 1898–1907 CrossRef CAS.
- M. M. Amini, A. Mohammadkhani and A. Bazgir, ChemistrySelect, 2018, 3, 1439–1444 CrossRef CAS.
- V. Kandathil, R. B. Dateer, B. Sasidhar, S. A. Patil and S. A. Patil, Catal. Lett., 2018, 6, 1562–1578 CrossRef.
- A. R. Hajipour and P. Abolfathi, Catal. Commun., 2018, 103, 92–95 CrossRef CAS.
- (a) M. Blosi, S. Ortelli, A. Costa, M. Dondi, A. Lolli, S. Andreoli, P. Benito and S. Albonetti, Materials, 2016, 9, 550–575 CrossRef PubMed; (b) G. Sharma, A. Kumar, S. Sharma, M. Naushad, R. P. Dwivedi, Z. A. ALOthman and G. T. Mola, J. King Saud Univ., Sci., 2017 DOI:10.1016/j.jksus.2017.06.012.
- R. Narayanan, Molecules, 2010, 15, 2124–2138 CrossRef CAS PubMed.
- R. K. Rai, D. Tyagi, K. Gupta and S. K. Singh, Catal. Sci. Technol., 2016, 6, 3341–3361 RSC.
- L. D. Pachón, M. B. Thathagar, F. Hartl and G. Rothenberg, Phys. Chem. Chem. Phys., 2006, 8, 151–157 RSC.
- A. Ohtaka, J. M. Sansano, C. Nájera, I. Miguel-García, Á. Berenguer-Murcia and D. Cazorla-Amorós, ChemCatChem, 2015, 7, 1841–1847 CrossRef CAS.
- Q. Xiao, S. Sarina, A. Bo, J. Jia, H. Liu, D. P. Arnold, Y. Huang, H. Wu and H. Zhu, ACS Catal., 2014, 4, 1725–1734 CrossRef CAS.
- T. Chatterjee, R. Dey and B. C. Ranu, New J. Chem., 2011, 35, 1103–1110 RSC.
- A. Grirrane, H. Garcia and A. Corma, J. Catal., 2013, 302, 49–57 CrossRef CAS.
- M. Planellas, Y. Moglie, F. Alonso, M. Yus, R. Pleixats and A. Shafir, Eur. J. Org. Chem., 2014, 3001–3008 CrossRef CAS.
- D. Gaikwad, K. Undale, D. Patil, D. Pore and A. Kamble, Res. Chem. Intermed., 2018, 44, 265–275 CrossRef CAS.
- R. Dey, K. Chattopadhyay and B. C. Ranu, J. Org. Chem., 2008, 73, 9461–9464 CrossRef CAS PubMed.
- D. Srimani, A. Bej and A. Sarkar, J. Org. Chem., 2010, 75, 4296–4299 CrossRef CAS PubMed.
- J. Song, X. Feng, Y. Yamamoto, A. I. Almansour, N. Arumugam, R. Suresh Kumar and M. Bao, Asian J. Org. Chem., 2017, 6, 177–183 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |