Open Access Article
Open Access ArticleAn efficient, mild and metal free L-proline catalyzed construction of fused pyrimidines under microwave conditions in water†
Manvendra S. Kaurava,
Pramod K. Sahu *ab,
Praveen K. Sahub,
Mouslim Messalic,
Saud M. Almutairid,
Puran L. Sahuef and
Dau D. Agarwalab
*ab,
Praveen K. Sahub,
Mouslim Messalic,
Saud M. Almutairid,
Puran L. Sahuef and
Dau D. Agarwalab
aSchool of Studies in Chemistry, Jiwaji University, Gwalior-474011, Madhya Pradesh, India. E-mail: sahu.chemistry@gmail.com; researchdata6@gmail.com
bDepartment of Industrial Chemistry, Jiwaji University, Gwalior-474011, Madhya Pradesh, India
cDepartment of Chemistry, Taibah University, 30002 Al-Madina Al-Mounawara, Saudi Arabia
dKing Abdulaziz City for Science and Technology, P. O. Box 6086, Riyadh 11442, Saudi Arabia
eIndian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Sector-23, Raj Nagar, Ghaziabad 201002, India
fNational Dope Testing Laboratory (NDTL), Ministry of Youth Affair & Sports, Government of India, J. L. N. Stadium Complex East Gate No. 10, Lodi Road, New Delhi-3, India
First published on 4th February 2019
Abstract
One-pot condensation of 4-hydroxy coumarins, aldehydes and urea/thiourea to build C–C and C–N bonds is described. Fused pyrimidines have been synthesized under mild reaction conditions using L-proline. The protocol has been performed rapidly and efficiently in water under metal free conditions. Heterocyclic derivatives have been synthesized using the present methodology and avoid the use of hazardous solvents over conventional organic solvents. A proposed mechanism could be established for three component reactions. The present study reveals the first case in which L-proline has been explored as a homogeneous catalyst in the synthesis of fused pyrimidines in water under microwave irradiation. This synthesis involves simple workup and acceptable efficiency. The most notable feature of this protocol is the ability of the catalyst to influence asymmetric induction in the reaction.
1. Introduction
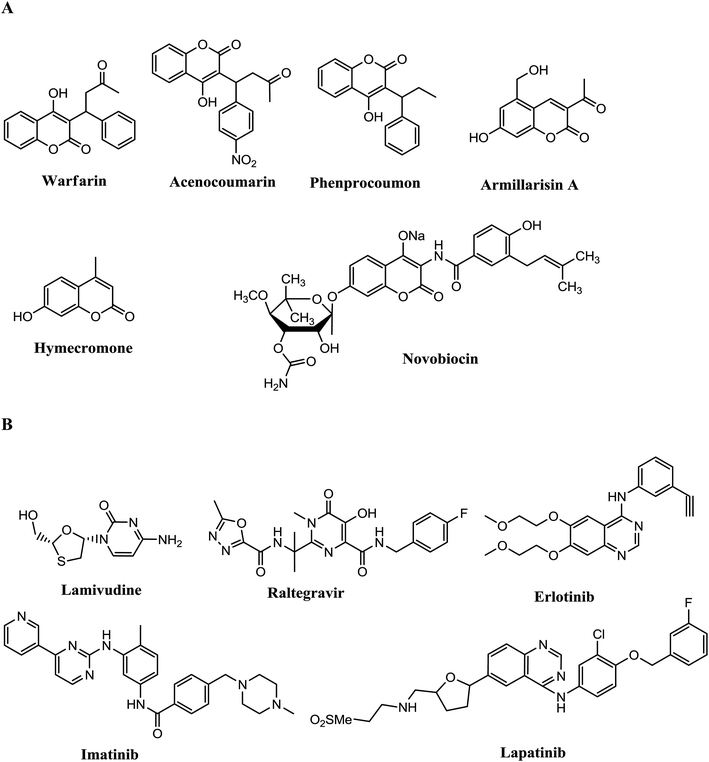
Multicomponent reactions (MCRs) have high efficiency and are a tool for development of different scaffolds for synthesis of many active drugs.1,2 In modern organic chemistry, the development of environmentally benign procedures in chemical and pharmaceutical industries has become a crucial and demanding research area. MRCs offer several advantages such as one-pot rather than multi-step synthesis of target compounds, and avoiding unnecessary expensive purification, toxic reagents and solvents.3 Proline is a chiral organo-catalyst having advantages over other catalyst such as being inexpensive, efficient and readily available.4 Proline act as an acid or a base which catalyzes chemical transformations similar to enzymatic catalysis.5 L-Proline has been effectively used in various organic transformations,6,7 direct catalytic asymmetric aldol, Mannish and Michael.8–10 Polysubstituted heterocyclic ortho-quinones,11 pyridines,12 acridine derivatives,13 pyrans and thiopyrans,14 and quinolines.15Coumarin moieties are involved in plants16 and showed anticoagulation, antiviral,17 anti-inflammatory,18 antibacterial19 and anticancer20 activities. Fused pyrimidines,21 chomenopyrimidine,22 and pyrimidines have also been reported as having anti-viral, anti-tumor, anti-inflammatory, and antihypertensive activities,23–25 as well as being calcium channel modulators26 and antimicrobial agents.27–29 Coumarin derivatives (Fig. 1A) have become drugs such as the anticoagulants warfarin,30a acenocoumarin,30b and phenprocoumon,30c all acting as vitamin K antagonists, the choleretics armillarisin A,31a hymecromone (umbelliferone),31b and the antibiotic novobiocin32 which is a potent inhibitor of bacterial DNA gyrase (GyrB). Some drugs such as Lamivudine,33a Raltegravir,33b Imatinib,34a,b Erlotinib34c,d and Lapatinib35 are types of drugs with pyrimidine core (Fig. 1B).
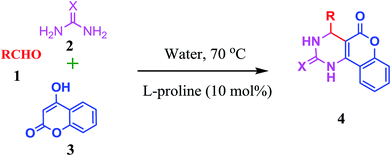
Thus as part of our research aimed at development of synthetic methodologies using environmentally benign catalysts through MCRs,36 we wish to report herein a metal free efficient and facile protocol for the three-component synthesis of fused pyrimidines in the presence of L-proline as an organo-catalyst in water at 70 °C, accompanied by moderate to good enantioselectivity (Scheme 1).
2. Materials and methods
2.1 Experimental
All reagents such as L-proline, 4-hydroxy coumarin, aldehydes etc. were analytical grade and have more than 98% purity. 1H and 13C NMR spectra were recorded on BRUKER AVANCE II 500 NMR spectrometer using CDCl3 and DMSO-d6 as solvent. Purity of the compound was checked by TLC. MS 1927 microwave starter kit was used for microwave reactions. Reaction was carried out under microwave conditions at 300 W in open to air conditions. E-Merck precoated TLC plates, RANKEM silica gel G for preparative thin-layer chromatography were used. Melting points were determined in open capillary and are uncorrected.2.2 Typical procedure for synthesis
In a 50 mL, 3 necks round bottom flask, charged appropriate aldehydes (5 mmol), 4-hydroxy coumarin (5 mmol) and urea/thiourea (5 mmol), water (10 mL) and L-proline (10 mol%). Stir the reaction mass and reflux at 70 °C. Reaction completion has monitored by TLC analysis. After reaction completion (monitoring by TLC), filtered the solid mass under vacuum then suck dried the solid and solid was recrystallized in ethanol.3. Results & discussion
Initially study has been started with screening of solvents in one-pot reaction 4-hydroxy coumarin (5 mmol), benzaldehyde (5 mmol) and urea (5 mmol). Reaction was efficiently promoted in water according to screening results as compared to other catalysts (Table 1, entry 1).| Entry | Solvents | Time (h) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 4-hydroxy coumarin (5 mmol), benzaldehyde (5 mmol) and urea (5 mmol) using L-proline (10 mol%). | |||
| 1 | Water | 3.0 | 90 |
| 2 | Toluene | 5.0 | 40 |
| 3 | DMF | 7.5 | 41 |
| 4 | Ethanol | 4.0 | 35 |
| 5 | Acetonitrile | 5.5 | 40 |
| 6 | THF | 5.0 | 53 |
Above screening results revealed that the solvent plays a key role in this transformation. For instance, a best yield was obtained when water was utilized as medium (Table 1, entry 1). Nevertheless, when other solvents, such as toluene, DMF, ethanol, acetonitrile and THF were employed, we observed average yield of 4a even after 7.5 h at 70 °C (Table 1, entries 2–6). Additionally, water is an eco-friendly, cheaper, safe solvent and preferred as medium for clean synthesis. In respect of solvent selection, water has been selected as solvent or aqueous medium. Subsequently, same reaction has been done with different catalysts and results are shown in Table 2. As indicated in Table 2, good yield was obtained in the presence of L-proline (Table 2, entry 3). However, other catalysts (such as p-TSA, TEA, CaCl2, H2SO4, sulphamic acid) have been afforded moderate yield with higher reaction time (Table 2, entries 5–9). In the screening part, we have examined acids, amine and metal salt as catalysts. However, all catalysts showed some activity but were not efficient. L-Proline has dual functionality and both free NH and COOH groups of L-proline are essential for efficient transformation. L-Proline easily form iminium complex, this may be due the fact that protonation of amine moiety of catalyst which subsequent easily react with aldehyde. Protonation of amine may be easily achieved after dipolar structure of acid and amine resultant high yield was obtained due to combine effect of acid and amine moiety. Rest of catalysts have not this dual functionality or nature to catalyze the reaction efficiently results low yield was achieved.
| Entry | Catalysts | Time (h) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 4-hydroxy coumarin (5 mmol), benzaldehyde (5 mmol) and urea (5 mmol) using water. | |||
| 1 | L-Proline (2 mol%) | 8.0 | 61 |
| 2 | L-Proline (5 mol%) | 4.0 | 77 |
| 3 | L-Proline (10 mol%) | 3.0 | 90 |
| 4 | L-Proline (15 mol%) | 3.0 | 90 |
| 5 | p-TSA (10 mol%) | 10.0 | 49 |
| 6 | TEA (10 mol%) | 8.0 | 59 |
| 7 | CaCl2 (10 mol%) | 10.0 | 60 |
| 8 | H2SO4 (10 mol%) | 160 | 70 |
| 9 | Sulphamic acid (10 mol%) | 180 | 42 |
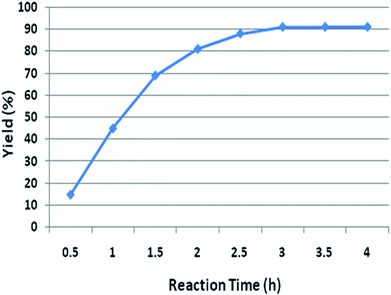
After screening of solvents and catalysts, loading of catalyst has been evaluated in one pot condensation (Table 2, entries 1–4). Screening results have shown that catalyst amount play a crucial role in completion of reaction. Excellent yield was obtained with 10 mol% of L-proline which could not be raised by increasing the catalyst loading. Accordingly, 10 mol% of catalyst loading was acceptable for this transformation. The reaction was then conducted at different time interval, such as 0.5 h, 1.0 h, 1.5 h, 2.0 h, 2.5 h, 3.0 h, 3.5 h and 4.0 h, to determine the optimum time for this transformation. It can be concluded that after 3 h time interval, highest yield (91%) was obtained (Fig. 2).
There are few reports in literature for synthesis of 3,4-dihydro-1H-chromeno[4,3-d]pyrimidine-2,5-dione/thione derivatives using 4-hydroxy coumarin, aldehydes and urea/thiourea in the presence of homogeneous and heterogeneous catalysts. Reported methods show that researchers has used acids such as HCl37–39,41,42 and chloro sulphonic acid37,40 which has drawbacks such as longer reaction time,37–39,41 harsh reaction conditions such as ultrasonication used,38,40,42,43 higher temperature (60 °C, 110 °C and reflux), hazardous solvent (MeOH, EtOH and ACN)37–39,41–44 and often lower yield.39–43 Although the ultrasonication technology has been shown feasible on a small scale, the commercialization of sonolysis is still a challenge due to its high energy requirement.45 On the other hand, vanadium chloride has been used as catalyst44 with lower yield, hazardous solvent (ACN) and higher temperature. Many studies have been revealed that exposure of vanadium may cause respiratory dysfunction,46 hematological and biochemical alterations, and renal toxicity47 reproductive and developmental toxicity immunotoxicity, mutagenicity48 and neurotoxicity may also occur.49 All above reported methods have at least one mentioned drawback resultant there is need to develop a methodology which remove all drawback in a single procedure. It is important to note that the previous above methods reported in the literature do not show any asymmetric induction, however target compound has a chiral centre. To solve this problem, L-proline was used as enantioselective organocatalyst in water as environmental benign solvent under microwave conditions and shown good enantioselectivity. Several advantages offered by this method such as its generality, simplicity, high yields and environmental friendly solvent used (Table 3).
| Entry | Catalyst | Solvent | Conditions | Time (h/min) | Yield (%) | eec | Reference |
|---|---|---|---|---|---|---|---|
| a Microwave conditions.b Ultrasonication.c ee = enantiomeric excess. | |||||||
| 1 | HCl/chloro sulphonic acid | MeOH | 60 °C | 8.0 h | 96 | — | 37 |
| 2 | HCl | EtOH | Reflux/MWa | 12 h | 94 | — | 38 |
| 3 | HCl | MeOH | Reflux | Overnight | 59 | — | 39 |
| 4 | Chloro sulphonic acid | — | 60 °C/USb | 30 min | 92 | — | 40 |
| 5 | HCl | EtOH | Reflux | 12 h | 74 | — | 41 |
| 6 | HCl/silica gel/acidic alumina/montmorillonite-K10 clay | MeOH | 110 °C/MWa | 4–6 min | 60/83/90/85 | — | 42 |
| 7 | K2CO3 | EtOH/H2O | Reflux/MWa | 7 h | 53 | — | 43 |
| 8 | VCl3 | Acetonitrile | Reflux | 2 h | 82 | — | 44 |
| 9 | L-Proline | Water | MWa | 10 min | 90 | 98 | Present work |
To explore the catalytic activity of L-proline, the scope the present methodology has been applied in the synthesis of various substituted fused pyrimidines. Different electron donating and electron withdrawing substituents have been investigated and results are incorporated in Table 4. From Table 4, the reaction was performed smoothly with para substituents and synthesized compounds have been characterized by spectroscopic analysis. Copy of all 1H and 13C NMR spectra is placed in the ESI† and confirmed the proposed structure of heterocycles.
| Entry | Product | Time (h/min) | Yield (%) | ee% | ||
|---|---|---|---|---|---|---|
| CHa | MWb | CH | MW | |||
| a CH = conventional heating.b MW = microwave conditions. | ||||||
| 1 | 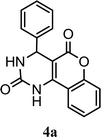 |
3.0 h | 10 min | 90 | 92 | 98 |
| 2 | 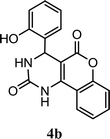 |
4.5 h | 8.0 min | 88 | 86 | 89 |
| 3 | 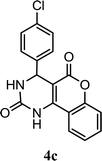 |
5.0 h | 10 min | 83 | 86 | 91 |
| 4 | 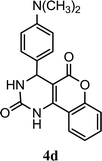 |
4.5 h | 10 min | 83 | 85 | 86 |
| 5 | 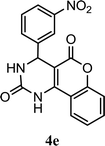 |
4.5 h | 8.0 min | 82 | 81 | 83 |
| 6 | 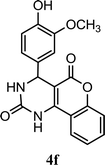 |
5.0 h | 5.0 min | 89 | 87 | 89 |
| 7 | 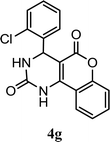 |
5.0 h | 10 min | 89 | 88 | 90 |
| 8 | 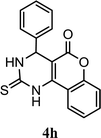 |
5.0 h | 10 min | 92 | 91 | 93 |
| 9 | 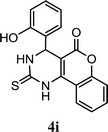 |
5.0 | 8.0 min | 93 | 92 | 96 |
| 10 | 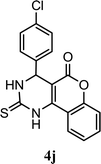 |
5.0 h | 10 min | 92 | 90 | 92 |
| 11 | 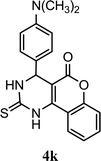 |
5.0 h | 10 min | 91 | 92 | 97 |
| 12 | 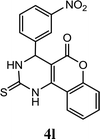 |
5.0 h | 8.0 min | 87 | 85 | 85 |
| 13 | 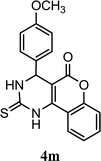 |
5.0 h | 5.0 min | 90 | 88 | 90 |
| 14 | 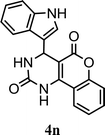 |
5.0 h | 5.0 min | 90 | 91 | 91 |
| 15 | 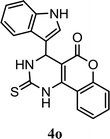 |
5.0 h | 8.0 min | 79 | 83 | 84 |
| 16 | 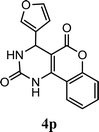 |
5.0 h | 5.0 min | 83 | 85 | 88 |
| 17 | 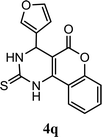 |
5.0 h | 10 min | 83 | 83 | 86 |
| 18 | 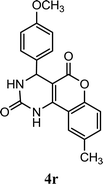 |
4.5 | 10 min | 80 | 83 | 84 |
| 19 | 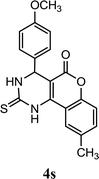 |
4.5 | 10 min | 81 | 82 | 89 |
| 20 | 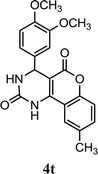 |
5.0 | 10 min | 77 | 81 | 85 |
Energy transfer depends on the thermal conductivity which is relatively slow and insufficient upon conventional heating resultant higher reaction time is required for completion of reaction. In contrast, microwave conditions are required minimum time to complete the reaction. Apart from this, the advantages of numerous microwave (MW) induced reactions over conventional reactions, and their utility in organic synthesis, have been fully recognized in the last two decades.50 To minimize the reaction time, reaction has performed under microwave conditions. The results showed that reaction has completed within 5–10 minutes with good yield under microwave conditions as compared to conventional heating. Therefore, microwave irradiation reducing the reaction time with good enantioselectivity (Table 4). The enantiomeric excess of the compounds synthesized was determined by employing chiral HPLC using OD-H column. Excellent enantioselectivity upto 98% ee was obtained (Table 4, entry 1). For the rest of the compounds enantiomeric excess was found to be in the range of to 83% ee to 97% ee. It is important to note that the previous methods reported in the literature do not show any asymmetric induction (Table 3).
Synthesized compounds 4a–4t were confirmed by spectroscopic analysis. 1H NMR of compound 4a showed characteristic signal at 6.36δ as singlet due to 4-H, multiplet for nine hydrogens of aromatic rings in the downfield region between 7.09–7.39δ and two singlet has been arised at 7.60 and 7.90 for –NH. Likewise derivatives 4d, 4k and 4f demonstrated the singlet at 3.11δ for six hydrogens of N(CH3)2 and singlet at 3.73δ for OCH3 respectively. 13C NMR of compound 4a showed characteristic signal at 36δ for C-1 carbon, signal at 104 has been assigned for C-2 carbon, other characteristic signal for ketonic carbon (C-4 and C-11) exhibited at 164δ and 165δ. Derivatives 4d, 4k and 4f have been showed characteristic signal at 45 and 56δ for –N(CH3)2 and OCH3 group. Mass spectra as well as elemental analysis also confirmed the structure of final product.
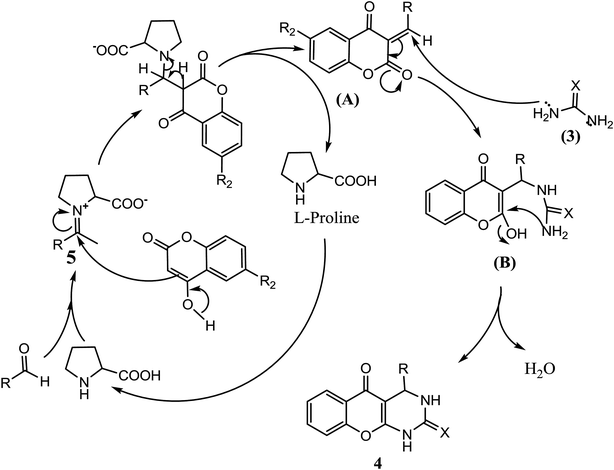
A plausible mechanism for reaction of 4-hydroxy coumarin (1), aldehydes (2), and urea/thiourea (3) to synthesis of fused pyrimidines (4) is depicted in Scheme 2. Based on literature, L-proline having dual functionality as acid and base can catalyze aldol related reactions such as Knoevenagel condensation as well as Michael addition.51 Previous study has shown that Knoevenagel condensation reaction efficiently catalyzed by amino acid catalyst and supports present mechanistic pathway.52 The reaction presumably proceeds through initial activation of the aldehyde by L-proline to form an iminium complex53 which further facilitates the Knoevenagel condensation to produce intermediate which pursue by Michael addition of urea/thiourea (3) on double bond of intermediate (A) to form intermediate (B). Furthermore, carbonyl and amino corner of the Michael adduct B was condensed through intramolecular cyclization to give desire target (4).
To support the plausible mechanism, proposed reaction intermediate (A) has been isolated and characterized. First of all reaction of 4-hydroxy coumarin and 2-hydroxy benzaldehyde has been carried out in optimized reaction conditions and formed the intermediate (A). Further intermediate (A) has been isolated and characterized by 1H and 13C NMR. Characterization data and literature have also been supported the structure of intermediate (A).54 Then isolated intermediate (A) was react with third component (urea) and achieved the product (4b). Present investigation has confirmed proposed mechanistic pathway by which target compound was achieved.
4. Conclusions
In conclusion, an enantioselective and metal free L-proline catalyzed protocol for the synthesis of fused pyrimidines with good yield in water as a green solvent using urea/thiourea, aldehydes and 4-hydroxy coumarin. Environmental benign one pot strategy has been explored with L-proline successfully which generate a green platform in future for enantioselective synthesis of novel molecules in water. Operational simplicity, metal-free approach, compatibility with various aldehydes and 4-hydroxy coumarin, simple se of workup, neat and clean synthesis are notable advantages of this protocol. In term of green solvent, an environmental benign solvent i.e. water was used which is very inexpensive and having reactivity and selectivity toward reaction media. Most notable feature of this methodology is enantioselective synthesis with more than 98% ee.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors are grateful thanks to the IPC Ghaziabad, India for performing 1H and 13C NMR spectra.References
- (a) R. Hajinasiri, Z. Hossaini and F. Sheikholeslami-Farahani, Comb. Chem. High Throughput Screen., 2015, 18, 42 CrossRef CAS; (b) Z. Hossaini, S. Soltani, F. Sheikholeslami-Farahani, S. Z. Sayyed-Alangi and H. Sajjadi-Ghotabadi, Chem. Heterocycl. Compd., 2015, 51, 26 CrossRef CAS; (c) Z. Hossaini, F. Rostami-Charati, M. Ghasemian and S. Afshari Sharif Abad, Synlett, 2015, 26, 1222 CrossRef CAS.
- (a) Z. Hossaini, F. Rostami-Charati, M. Ghambarian and S. A. Siadati, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 1177 CrossRef CAS; (b) P. Slobbe, E. Ruijter and R. V. A. Orru, Med. Chem. Commun., 2012, 3, 1189 RSC.
- (a) V. D. G. Heijden, E. Ruijter and R. V. A. Orru, Synlett, 2013, 666 Search PubMed; (b) C. Hulme, M. Ayaz, G. Martinez-Ariza, F. Medda and A. Shaw, in Small molecule medicinal chemistry: strategies and technologies, ed. W. Czechtizky and P. Hamley, Wiley-VCH, Weinheim, 2015, ch. 6 Search PubMed; (c) A. Gollner, Synlett, 2015, 426 CrossRef CAS.
- (a) B. List, R. A. Lerner and C. F. Barbas, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS; (b) A. Cordova, W. Notz and C. F. Barbas, Chem. Commun., 2002, 3024 RSC; (c) N. S. Chowdari, D. B. Ramachary and C. F. Barbas, Synlett, 2003, 1906 CAS.
- (a) S. Balalaie, M. Bararjanian, A. M. Amani and B. Movassagh, Synlett, 2006, 263 CrossRef CAS; (b) P. Kotrusz and S. Toma, ARKIVOC, 2006, v, 100 Search PubMed; (c) J. Mabry and B. Ganem, Tetrahedron Lett., 2006, 47, 55 CrossRef CAS; (d) S. Chandrasekher, K. Vijeender and V. K. Reddy, Tetrahedron Lett., 2005, 46, 6991 CrossRef; (e) S. Chandrasekhar, C. Narsihmulu, N. R. K. Reddy and S. S. Sultana, Tetrahedron Lett., 2004, 45, 4581 CrossRef CAS; (f) T. Darbre and M. Machuqueiro, Chem. Commun., 2003, 1090 RSC.
- (a) Y. Wang, Z. C. Shang, T. X. Wu, J. C. Fan and X. Chen, J. Mol. Catal. A: Chem., 2006, 253, 212 CrossRef CAS; (b) M. Srinivasan, S. Perumal and S. Selvaraj, ARKIVOC, 2005, xi, 201 Search PubMed; (c) G. Sabitha, N. Fatima, E. V. Reddy and J. S. Yadav, Adv. Synth. Catal., 2005, 347, 1353 CrossRef CAS; (d) R. Dodda and C. G. Zhao, Synthesis, 2006, 19, 3238 Search PubMed.
- (a) R. Varala, E. Ramu, N. Sreelatha and S. R. Adapa, Tetrahedron Lett., 2006, 476, 877 CrossRef; (b) R. Varala and S. R. Adapa, Org. Process Res. Dev., 2005, 9, 853 CrossRef CAS; (c) Z. An, W. Zhang, H. Shi and J. He, J. Catal., 2006, 241, 319 CrossRef CAS; (d) N. N. Karade, V. H. Budhewar, S. V. Shinde and W. N. Jadhav, Lett. Org. Chem., 2007, 4, 16 CrossRef CAS.
- B. Alcaide, P. Almendros, A. Luna and M. R. Torres, J. Org. Chem., 2006, 71, 4818 CrossRef CAS PubMed.
- (a) J. M. Janey, Y. Hsiao and J. D. Armstrong, J. Org. Chem., 2006, 71, 390 CrossRef CAS PubMed; (b) B. List, P. Pojarliev, W. T. Biller and H. J. Martin, J. Am. Chem. Soc., 2002, 124, 827 CrossRef CAS PubMed.
- (a) M. S. Rasalkar, M. K. Potdar, S. S. Mohile and M. M. Salunkhe, J. Mol. Catal. A: Chem., 2005, 235, 267 CrossRef CAS; (b) P. Kotrusz and S. Toma, Molecules, 2006, 11, 197 CrossRef CAS PubMed; (c) P. Kotrusz and S. Toma, ARKIVOC, 2006, 100 Search PubMed.
- S. M. Rajesh, B. D. Bala, S. Perumal and J. M. Menéndez, Green Chem., 2011, 13, 3248 RSC.
- (a) B. Janardhan, V. Ravibabu, P. A. Crooks and B. Rajitha, Org. Commun., 2012, 5, 186 CAS; (b) C. Mukhopadhyay, P. K. Tapaswi and R. J. Butcher, Tetrahedron Lett., 2010, 51, 1797 CrossRef CAS.
- M. R. P. Heravi and P. Aghamohammadi, C. R. Chim., 2012, 15, 448 CrossRef.
- (a) N. M. H. Elnagdi and N. S. Al-Hokbany, Molecules, 2012, 17, 4300 CrossRef CAS PubMed; (b) P. P. Bora, M. Bihani and G. Bez, RSC Adv., 2015, 5, 50597 RSC.
- S. Karamthulla, S. Pal, T. Parvin and L. H. Choudhury, RSC Adv., 2014, 4, 15319 RSC.
- (a) R. D. H. Murray, J. Mendez and S. A. Brown, Chemistry and Biochemistry, John Wiley & Sons Ltd, New York, 1982, p. 21 Search PubMed; (b) M. M. Garazd, Y. L. Garadz and V. P. Khilya, Chem. Nat. Compd., 2003, 39, 54 CrossRef CAS; (c) R. D. H. Murray, Nat. Prod. Rep., 1995, 12, 477 RSC; (d) A. Estvez-Braun and A. G. Gonzalez, Nat. Prod. Rep., 1997, 14, 465 RSC.
- (a) J. R. Hwu, R. Singha, S. C. Hong, Y. H. Chang, A. R. Das, I. Vliegen, E. D. Clercq and J. Neyts, Antiviral Res., 2008, 77, 157 CrossRef CAS PubMed; (b) J. Neyts, E. D. Clercq, R. Singha, Y. H. Chang, A. R. Das, S. K. Chakraborty, S. C. Hong, S. C. Tsay, M. H. Hsu and J. R. Hwu, J. Med. Chem., 2009, 52, 1486 CrossRef CAS PubMed; (c) J. R. Hwu, S. Y. Lin, S. C. Tsay, E. D. Clercq, P. Leyssen and J. Neyts, J. Med. Chem., 2011, 54, 2114 CrossRef CAS PubMed.
- (a) Z. P. Li, J. F. Hu, M. N. Sun, H. J. Ji, S. F. Chu, G. Liu and N. H. Chen, Int. Immunopharmacol., 2012, 14, 145 CrossRef CAS PubMed; (b) Z. P. Li, J. F. Hu, M. N. Sun, H. J. Ji, M. Zhao, D. H. Wu, G. Y. Li, G. Liu and N. H. Chen, Eur. J. Pharmacol., 2011, 661, 118 CrossRef CAS PubMed.
- Y. Shi and C. H. Zhou, Bioorg. Med. Chem. Lett., 2011, 21, 956 CrossRef CAS PubMed.
- (a) F. Meggio, M. A. Pagano, S. Moro, G. Zagotto, M. Ruzzene, S. Sarno, G. Cozza, J. Bain, M. Elliott, A. D. Deana, A. M. Brunati and L. A. Pinna, Biochemistry, 2004, 43, 12931 CrossRef CAS PubMed; (b) A. Chilin, R. Battistutta, A. Bortolato, G. Cozza, S. Zanatta, G. Poletto, M. Mazzorana, G. Zagotto, E. Uriarte, A. Guiotto, L. A. Pinna, F. Meggio and S. Moro, J. Med. Chem., 2008, 51, 752 CrossRef CAS PubMed; (c) G. L. Bras, C. Radanyi, J. F. Peyrat, J. D. Brion, M. Alami, V. Marsaud, B. Stella and J. M. Renoir, J. Med. Chem., 2007, 50, 6189 CrossRef PubMed; (d) H. P. Zhao, A. C. Donnelly, B. R. Kusuma, G. E. L. Brandt, D. Brown, R. A. Rajewski, G. Vielhauer, J. Holzbeierlein, M. S. Cohen and B. S. J. Blagg, J. Med. Chem., 2011, 54, 3839 CrossRef CAS PubMed; (e) H. P. Zhao, B. Yan, L. B. Peterson and B. S. J. Blagg, ACS Med. Chem. Lett., 2012, 3, 327 CrossRef CAS PubMed; (f) A. C. Donnelly, J. R. Mays, J. A. Burlison, J. T. Nelson, G. Vielhauer, J. Holzbeierlein and B. S. J. Blagg, J. Org. Chem., 2008, 73, 8901 CrossRef CAS PubMed; (g) A. Purohit, L. W. L. Woo, B. V. L. Potter and M. J. Reed, Cancer Res., 2000, 60, 3394 CAS; (h) L. W. L. Woo, A. Purohit, B. Malini, M. J. Reed and B. V. L. Potter, Chem. Biol., 2000, 7, 773 CrossRef CAS PubMed; (i) B. Malini, A. Purohit, D. Ganeshapillai, L. W. L. Woo, B. V. L. Potter and M. J. Reed, J. Steroid Biochem. Mol. Biol., 2000, 75, 253 CrossRef CAS.
- J. Kempson, W. J. Pitts, J. Barbosa, J. Guo, O. Omotoso, A. Watson, K. Stebbins, G. C. Starling, J. H. Dodd, J. C. Barrish, R. Felix and K. Fischer, Bioorg. Med. Chem. Lett., 2005, 15, 1829 CrossRef CAS PubMed.
- O. Bruno, C. Brullo, S. Schenone, F. Bondavalli, A. Ranise, M. Tognolini, M. Impicciatore, V. Ballabeni and E. Barocelli, Bioorg. Med. Chem., 2006, 14, 121 CrossRef CAS PubMed.
- (a) N. O. Al-Harbi, S. A. Bahashwan, A. A. Fayed, M. S. Aboonq and A. E. E. Amr, Int. J. Biol. Macromol., 2013, 57, 165 CrossRef CAS PubMed; (b) E. Petersen and D. R. Schmidt, Expert Rev. Anti-Infect. Ther., 2003, 1, 175 CrossRef CAS PubMed.
- (a) E. Nadal and E. Olavarria, Int. J. Clin. Pract., 2004, 58, 511 CrossRef CAS PubMed; (b) B. S. Dixon, G. J. Beck, M. A. Vazquez, A. Greenberg, J. A. Delmez, M. Allon, L. M. Dember, J. Himmelfarb, J. J. Gassman, T. Greene, M. K. Radeva, I. J. Davidson, T. A. Ikizler, G. L. Braden, A. Z. Fenves, J. S. Kaufman, J. R. Cotton Jr, K. J. Martin, J. W. McNeil, A. Rahman, J. H. Lawson, J. F. Whiting, B. Hu, C. M. Meyers, J. W. Kusek and H. I. Feldman, N. Engl. J. Med., 2009, 360, 2191 CrossRef CAS PubMed.
- S. V. Dinakaran, B. Bhargavi and K. K. Srinivasan, Der Pharma Chemica, 2012, 4, 255 Search PubMed.
- (a) G. C. Rovnyak, S. D. Kimbal, B. Beyer, G. Cucinotta, J. D. Dimarco, J. Gougoutas, A. Hedberg, M. Malley, J. P. MaCaethy, R. Zhang and S. Mereland, J. Med. Chem., 1995, 38, 119 CrossRef CAS PubMed; (b) C. O. Kappe, W. M. F. Fabian and M. A. Semons, Tetrahedron, 1997, 53, 2803 CrossRef CAS.
- K. R. Lanjewar, A. M. Rahatgaonkar, M. S. Chorghade and B. D. Saraf, Indian J. Chem., 2009, 48B, 1732 CAS.
- (a) M. M. Heravi, L. Ranjwar, F. Deriknand, B. Alimadadi and H. A. Oskooie, Mol. Diversity, 2008, 12, 181 CrossRef CAS PubMed; (b) S. Chang, J. S. Ji and L. Yu, J. Chin. Chem. Soc., 2008, 55, 292 CrossRef; (c) N. K. Shah, M. P. Patel and R. G. Patel, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2704 CrossRef CAS.
- R. Kumar, S. Malik and R. Chamdra, Indian J. Chem., 2009, 48B, 718 CAS.
- (a) Y. Liu, S. Liu, Y. Shi, M. Qin, Z. Sun and G. Liu, Xenobiotica, 2017, 48, 818 CrossRef; (b) S. S. Rathore, S. K. Agarwal, S. Pande, S. K. Singh, T. Mittal and B. Mittal, PLoS One, 2012, 7, e37844 CrossRef CAS; (c) J. H. Prochaska, S. Göbel, K. Keller, M. Coldewey, A. Ullmann, H. Lamparter, C. Jünger, Z. Al-Bayati, C. Baer, U. Walter, C. Bickel, H. t. Cate, T. Münzel and P. S. Wild, BMC Med., 2015, 13, 14 CrossRef PubMed.
- (a) J. B. Zhu, L. B. Luan and Q. C. Shi, Yaoxue Xuebao, 1992, 27, 231 CAS; (b) A. Abate, V. Dimartino, P. Spina, P. L. Costa, C. Lombardo, A. Santini, M. Del Piano and P. Alimonti, Drugs Exp. Clin. Res., 2001, 27, 223 CAS.
- V. Rodríguez-Cerrato, G. Del Prado, L. Huelves, P. Naves, V. Ruiz, E. García, C. Ponte and F. Soriano, Int. J. Antimicrob. Agents, 2010, 35, 544 CrossRef.
- (a) S. L. Gaonkar and H. Shimizu, Tetrahedron, 2010, 66, 3314 CrossRef CAS; (b) T. L. Gilchrist, Heterocyclic Chemistry, John Wiley & Sons, 1997 Search PubMed.
- (a) R. H. Parker and W. M. Jones, J. Org. Chem., 1978, 43, 2548 CrossRef CAS; (b) R. H. Wiley and N. R. Smith, Organic Syntheses; Collect, John Wiley & Sons, New York, 1963, vol. 4, p. 201 Search PubMed; (c) H. von Pechmann, Justus Liebigs Ann. Chem., 1891, 264, 261 CrossRef; (d) I. W. Ashworth, M. C. Bowden, B. Dembofsky, D. Levin, W. Moss, E. Robinson, N. Szczur and J. Virica, Org. Process Res. Dev., 2003, 7, 74 CrossRef CAS.
- X. Li, C. Abell, B. H. Warrington and M. Ladlow, Org. Biomol. Chem., 2003, 1, 4392 RSC.
- (a) P. K. Sahu, P. K. Sahu, S. K. Gupta, D. Thavaselvam and D. D. Agarwal, Eur. J. Med. Chem., 2012, 54, 366 CrossRef CAS; (b) P. K. Sahu, P. K. Sahu, D. Thavaselvam, A. M. Alafeefy and D. D. Agarwal, Med. Chem. Res., 2015, 24, 725 CrossRef CAS; (c) P. K. Sahu, P. K. Sahu and D. D. Agarwal, RSC Adv., 2013, 3, 9854 RSC; (d) P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Ind. Eng. Chem. Res., 2014, 53, 2085 CrossRef CAS; (e) P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Catal. Sci. Technol., 2013, 3, 1520 RSC; (f) P. K. Sahu, P. K. Sahu and D. D. Agarwal, RSC Adv., 2014, 4, 40414 RSC; (g) P. K. Sahu, P. K. Sahu, Y. Sharma and D. D. Agarwal, J. Heterocycl. Chem., 2014, 51, 1193 CrossRef CAS; (h) P. K. Sahu, P. K. Sahu, M. S. Kaurav, M. Messali, S. M. Almutairi, P. L. Sahu and D. D. Agarwal, RSC Adv., 2018, 8, 33952 RSC; (i) P. K. Sahu, P. K. Sahu, M. S. Kaurav, M. Messali, S. M. Almutairi, P. L. Sahu and D. D. Agarwal, ACS Omega, 2018, 3, 15035 CrossRef CAS; (j) P. K. Sahu, P. K. Sahu and D. D. Agarwal, J. Indian Chem. Soc., 2015, 92, 169 CAS.
- D. Bhut, R. Gami, A. Parikh, C. Sharma and P. Patel, Pharma Sci. Monit., 2015, 6, 149 CAS.
- M. Kidwai, S. Saxena and R. Mohan, Russ. J. Org. Chem., 2006, 42, 52 CrossRef CAS.
- D. I. Brahmbhatt, G. B. Raolji, S. U. Pandya and U. R. Pandya, Indian J. Chem., 1999, 38(B), 839 Search PubMed.
- M. A. Abdulkarim Al-Kadasi and G. M. Nazeruddin, J. Chem. Pharm. Res., 2013, 5, 204 Search PubMed.
- P. K. Ambre, R. R. S. Pissurlenkar, R. D. Wavhale, M. S. Shaikh, V. M. Khedkar, B. Wan, S. G. Franzblau and E. C. Coutinho, Med. Chem. Res., 2014, 23, 2564 CrossRef CAS.
- M. Kidwai and P. Sapra, Synth. Commun., 2002, 32, 1639 CrossRef CAS.
- M. Kidwai, Priya and S. Rastogi, Z. Naturforsch., 2008, 63b, 71 Search PubMed.
- G. Sabitha, G. S. K. K. Reddy, K. B. Reddy and J. S. Yadav, Tetrahedron Lett., 2003, 44, 6497 CrossRef CAS.
- J. C. Crittenden, R. R. Trussell, D. W. Hand and G. Tchobanglouse, Water treatment principle and design, John Wily and Sons, 2nd edn, 2004 Search PubMed.
- M. A. Woodin, Y. Liu, D. Neuberg, R. Hauser and T. J. Smith, et al., Am. J. Ind. Med., 2000, 37, 353 CrossRef CAS.
- H. Zaporowska, W. Wasilewski and M. Sŀotwińska, BioMetals, 1993, 6, 3 CrossRef CAS.
- M. R. Avila-Costa, E. Montiel Flores, L. Colin-Barenque, J. L. Ordoñez and A. L. Gutiérrez, et al., Neurochem. Res., 2004, 29, 1365 CrossRef CAS.
- H. Li, D. Zhou, Q. Zhang, C. Feng and W. Zheng, et al., NeuroToxicology, 2013, 36, 49 CrossRef CAS PubMed.
- (a) L. Perreux and A. Loupy, Tetrahedron, 2001, 57, 9199 CrossRef CAS; (b) P. Lindström, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225 CrossRef; (c) A. Loupy, A. Petit, J. Hamelin, F. T. Boullet, P. Jacquault and D. Mathe, Synthesis, 1998, 1213 CrossRef CAS.
- (a) S. Chandrasekhar, C. Narsihmulu, N. R. K. Reddy and S. S. Sultana, Tetrahedron Lett., 2004, 45, 4581 CrossRef CAS; (b) P. Kotrusz and S. Toma, ARKIVOC, 2006, v, 100 Search PubMed; (c) T. Darbre and M. Machuqueiro, Chem. Commun., 2003, 7, 1090 RSC.
- (a) D. B. Ramachary and M. Kishor, Org. Biomol. Chem., 2010, 8, 2859 RSC; (b) D. B. Ramachary, M. A. Pasha and G. Thirupathi, Angew. Chem., Int. Ed., 2017, 56, 12930 CrossRef CAS PubMed; (c) R. Madhavachary and D. B. Ramachary, Eur. J. Org. Chem., 2014, 7317 CrossRef CAS; (d) D. B. Ramachary and S. Jain, Org. Biomol. Chem., 2011, 9, 1277 RSC; (e) D. B. Ramachary, M. Kishor and Y. V. Reddy, Eur. J. Org. Chem., 2008, 975 CrossRef CAS; (f) D. B. Ramachary and Y. V. A. Reddy, J. Org. Chem., 2010, 75, 74 CrossRef CAS PubMed; (g) D. B. Ramachary and M. Kishor, J. Org. Chem., 2007, 72, 5056 CrossRef CAS PubMed; (h) D. B. Ramachary, M. Kishor and R. G. Babul, Org. Biomol. Chem., 2006, 4, 1641 RSC.
- C. Mukhopadhyay, P. K. Tapaswi and R. J. Butcher, Tetrahedron Lett., 2010, 51, 1797 CrossRef CAS.
- M. Khoobi, A. Foroumadi, S. Emami, M. Safavi, G. Dehghan, B. H. Alizadeh, A. Ramazani, S. K. Ardestani and A. Shafiee, Chem. Biol. Drug Des., 2011, 78, 580 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07517d |
| This journal is © The Royal Society of Chemistry 2019 |