N-Heterocyclic carbene catalysed umpolung reactions of imines approaching enantioselective synthesis
Abstract
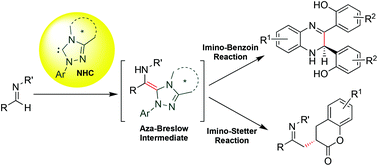
N-Heterocyclic carbenes (NHCs) have emerged as efficient catalysts for promoting umpolung reactions of aldehydes and enals. To a certain extent, the NHC catalyzed umpolung reactions of Michael acceptors are also reported. Recently, the NHCs have been successfully used for catalysing the umpolung reactions of aldimines through the formation of aza-Breslow intermediates. The initial reports on the chiral NHC-catalysed enantioselective umpolung reactions of imines are turning out to be very promising to achieve a high level of enantio-induction. This highlight article focuses on the NHC catalysed umpolung reactions of aldimines, with a special emphasis on the corresponding enantioselective transformations.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...