Synthesis of photolabile protecting group (PPG) protected uronic acid building blocks: applications in carbohydrate synthesis with the assistance of a continuous flow photoreactor†
Abstract
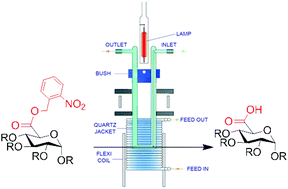
Photolabile protecting group (PPG) protected uronic acid building blocks were prepared and used for carbohydrate synthesis. Deprotection of the photolabile protecting group was achieved very efficiently by employing a continuous flow photoreactor under neutral conditions. Many conventional protecting groups were found to be stable during the photo-cleavage of the 2-nitrobenzyl group at 355 nm in methanol.


 Please wait while we load your content...
Please wait while we load your content...