Open Access Article
Open Access ArticleOxidative selenofunctionalization of allenes: convenient access to 2-(phenylselanyl)-but-2-enals and 4-oxo-3-(phenylselanyl)pent-2-enoates†‡
Benito
Alcaide
 *a,
Pedro
Almendros
*a,
Pedro
Almendros
 *b,
Teresa
Martínez del Campo
*b,
Teresa
Martínez del Campo
 a,
Laura
Martín
a,
Guillermo
Palop
a and
Mireia
Toledano-Pinedo
a
a,
Laura
Martín
a,
Guillermo
Palop
a and
Mireia
Toledano-Pinedo
a
aGrupo de Lactamas y Heterociclos Bioactivos, Departamento de Química Orgánica I, Unidad Asociada al CSIC, Facultad de Química. Universidad Complutense de Madrid, 28040 Madrid, Spain. E-mail: alcaideb@quim.ucm.es
bInstituto de Química Orgánica General, Consejo Superior de Investigaciones Científicas, IQOG-CSIC, Juan de la Cierva 3, 28006 Madrid, Spain. E-mail: Palmendros@iqog.csic.es
First published on 24th May 2019
Abstract
The controlled preparation of two types of α-seleno-α,β-unsaturated carbonyls, namely, α-selenoenals and α-selenoenones, has been accomplished directly from allenes through metal-free oxidative selenofunctionalization reactions. The decisive role of organoselenium and 1-fluoropyridinium reagents has been disclosed. The divergent reactivity due to the presence or absence of an ethoxycarbonyl moiety at the allene end has also been studied. A tentative pathway implying selective electrophilic addition of the selenium reagent to the allene moiety followed by adventitious water attack and concomitant oxidation has been proposed.
Introduction
Allenes (1,2-dienes) have attracted considerable interest in chemistry research due to their availability and unique reactivity, which allows for the selective synthesis of different functionalized organic molecules.1 On the other hand, the organoselenium motif is the central structural element of a variety of biologically active molecules.2 Besides, selenium-containing organic compounds are attractive to medicinal chemists because organic molecules bearing selenated moieties can enhance their pharmacological properties.3 As a result, several synthesis protocols toward organoselenium derivatives have been described starting from allenes.4 Inspired by the recently described organoselenium-mediated C–H pyridination of 1,3-dienes using 1-fluoropyridinium reagents (Scheme 1a),5 we decided to test the related C–H functionalization of 1,2-dienes. However, we did observe the selective formation of α-selenoenals and α-selenoenones (Scheme 1b),6 probably via the controlled electrophilic addition of the selenium reagent to the allene moiety followed by adventitious water attack and concomitant oxidation. 1-Fluoropyridinium compounds7 as oxidative functionalization reagents have several advantages over classical oxidants based on heavy metals, such as low toxicity and high functional group tolerance. Herein we report this appealing reactivity which allows one to obtain two different types of α-seleno-α,β-unsaturated carbonyls from allenes.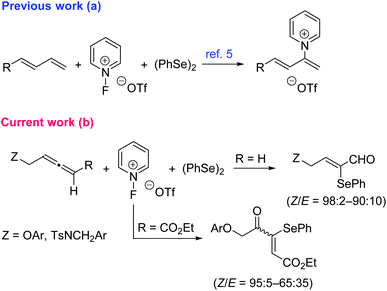 | ||
| Scheme 1 Previous report and the current study of the reactivity of dienes with the 1-fluoropyridinium/1,2-diphenyldiselane system. | ||
Results and discussion
Starting materials, allenes 1a–h unsubstituted at the proximal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and allenoates 2a–f, were prepared from common terminal alkyne precursors using the Crabbé reaction8 or treatment with ethyl diazoacetate,9 respectively (Scheme 2 and see the ESI for details‡).
C double bond and allenoates 2a–f, were prepared from common terminal alkyne precursors using the Crabbé reaction8 or treatment with ethyl diazoacetate,9 respectively (Scheme 2 and see the ESI for details‡).
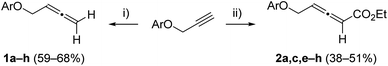 | ||
Scheme 2 Synthesis of allene precursors. (i) (CH2O)n, iPr2NH, CuBr, dioxane, reflux, 2 h. (ii) N2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Et, Et3N, CuI, CH3CN, rt. CHCO2Et, Et3N, CuI, CH3CN, rt. | ||
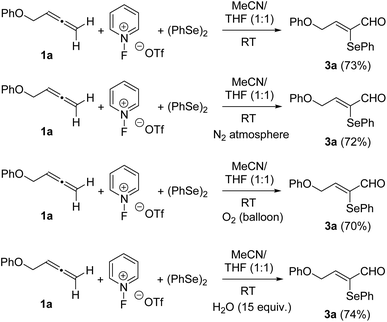
To screen the reactivity of the allene moiety towards C–H pyridination, terminal allene 1a was selected as the model substrate. In the event, the incorporation of the pyridine nucleus did not occur and α-phenylseleno-α,β-unsaturated aldehyde 3a was formed instead regio- and stereoselectively in 73% yield (Scheme 3). Next, we examined the reaction scope by varying the substitution of the arene moiety. Several substituents of different electronic demands were well tolerated. All the reactions progressed satisfactorily to provide the required α-selenoenals 3b–h as almost single (Z)-isomers in fair yields (Scheme 3).10 However, electron-withdrawing substituents at the arene moiety were counterproductive for the process, as 3- and 4-nitrobenzene provided the required products 3b and 3d in diminished yields. The presence of bulky substituents at the ortho-position such as in 3e and 3f was also unfavourable for the reaction yields. Taking into account the presence of both a PhSe group and a carbaldehyde functionality in adducts 3,11 we decided to modify the reaction conditions. The replacement of (PhSe)2 with PhSeCl yielded a chromatographically separable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (Z)- and (E)-(1-chloro-4-aryloxybut-2-en-2-yl)(phenyl)selanes 4 (Scheme 3). The use of alternative oxidants such as Oxone or Selectfluor resulted in complicated reaction mixtures, thus highlighting the important role of the 1-fluoropyridinium reagent.
1 mixture of (Z)- and (E)-(1-chloro-4-aryloxybut-2-en-2-yl)(phenyl)selanes 4 (Scheme 3). The use of alternative oxidants such as Oxone or Selectfluor resulted in complicated reaction mixtures, thus highlighting the important role of the 1-fluoropyridinium reagent.
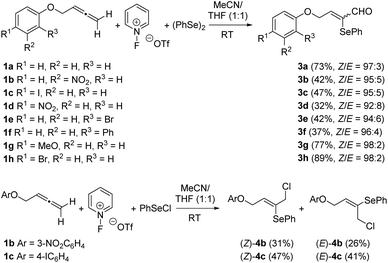 | ||
| Scheme 3 Synthesis of α-phenylseleno-α,β-unsaturated aldehydes 3 through the oxidative selenofunctionalization of allenes 1. | ||
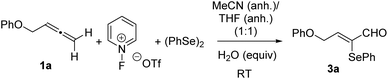
Owing to the structure of adducts 3, we suspect the involvement of adventitious water. Aiming to evaluate the effect of water on this transformation, strictly dehydrated acetonitrile and tetrahydrofuran were used as solvents followed by the controlled addition of water (Table 1). The use of rigorously anhydrous solvents resulted in almost the absence of reaction (entry 1, Table 1), while the addition of 1 equiv. of water produced a diminished yield (26%) of 3a (entry 2, Table 1). The addition of the high amounts of water was beneficial and gave rise to product 3a in reasonable yields (entries 3–5, Table 1). However, the alternative participation of oxygen from the air could not be discarded. To gain some insights into the reaction mechanism, control experiments were carried out. When the reaction of allene 1a was conducted under a N2 atmosphere under otherwise identical conditions, the yield remained almost unchanged (Scheme 4). Similar yields were obtained with the inclusion of an O2 balloon. The addition of 15 equivalents of water to the standard reaction resulted in almost no variation in the isolated yield of 3a (Scheme 4), which unquestionably proved that the amount of water available in regular solvents12 was enough for the reaction to proceed efficiently. From the above results, it is apparent that the carbaldehyde oxygen of 3a came from water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
A possible mechanistic pathway for the formation of α-selenoenals 3 from allenes 1 is depicted in Scheme 5. Initially, the generation of the electrophilic species PhSe(OTf) takes place through the oxidation of (PhSe)2 promoted by 1-fluoropyridinium triflate. Next, the discriminating reaction with the terminal double bond of the allene moiety of allenes 1 provides intermediate 1-phenylseleniran-1-iums 5, which after regioselective nucleophilic water attack are converted into 2-(phenylselanyl)but-2-en-1-ols 6. Although merely speculative at this time, the origin of the detected stereoselectivity may be due to the favourable syn arrangement of the PhSe and CH2OAr moieties with the latter acting as a directing group. The final oxidation of the hydroxyl functionality assisted by the 1-fluoropyridinium reagent provides α-phenylseleno-α,β-unsaturated aldehydes 3.
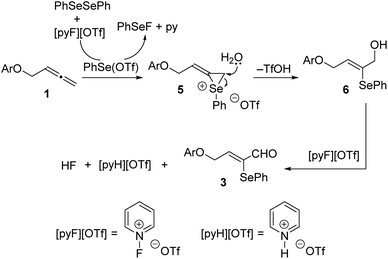 | ||
| Scheme 5 Proposed reaction pathway for the 1-fluoropyridinium triflate-assisted formation of α-selenoenals 3. | ||
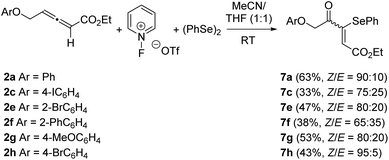
The oxidative selenofunctionalization of ethoxycarbonyl-allenes 2 was then surveyed under the optimized reaction conditions for the formation of α-selenoenals 3. In the event, the regioselectivity was totally reverted. The reactions proceeded to form the corresponding 4-oxo-3-(phenylselanyl)pent-2-enoates 7 in fair yields and good to moderate stereoselectivities (Scheme 6). In every single case, a ketone group was formed and placed at the former carbon(sp2) of the distal allene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.13 Contrary to α-selenoenals 3, products 7 were not formed as almost single (Z)-geometrical isomers.10 We suspected that the presence of the ethoxycarbonyl moiety brings about a different stereoelectronic environment which makes the selenofunctionalization and further water attack difficult, resulting in a lower stereoselectivity.
C double bond.13 Contrary to α-selenoenals 3, products 7 were not formed as almost single (Z)-geometrical isomers.10 We suspected that the presence of the ethoxycarbonyl moiety brings about a different stereoelectronic environment which makes the selenofunctionalization and further water attack difficult, resulting in a lower stereoselectivity.
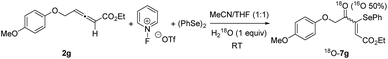
Based on the mechanistic proposal of Scheme 5, a plausible pathway for the formation of adducts 7 is depicted in Scheme 7. Initially, the electrophilic attack of the benzeneselenyl species to the more electron-rich allene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond takes place regioselectively to generate phenylseleniran-1-iums 8. A subsequent nucleophilic water attack can be produced at either carbon of the cations 8, but the site-selectivity is total and seleno-functionalized allylic alcohols 9 are exclusively formed. Probably, the formation of intermediates 9 is also the (Z)/(E)-stereodetermining step. Oxidation of the hydroxyl group at intermediates 9 provides final products 7 and releases pyridin-1-ium triflate and hydrogen fluoride. Alternatively, the hydroxy group of intermediates 9 may arise not from external water, but from the ester group of starting allenes 2 based on the proposal by Fu and Ma.4f To clarify the effective engagement of external water in the selenofunctionalization of ethoxycarbonyl-allenes, an 18O-labeling experiment was performed (Scheme 8). Hence, the reaction of allene 2g was carried out under otherwise optimal conditions, but in the presence of one equivalent of H218O with an isotope abundance of 97%. In the event, the resulting 4-oxo-3-(phenylselanyl)pent-2-enoate 7g was 18O-labeled (ca. 50%) as detected by mass spectrometry (see the ESI‡), suggesting the involvement of adventitious water in this transformation.
C double bond takes place regioselectively to generate phenylseleniran-1-iums 8. A subsequent nucleophilic water attack can be produced at either carbon of the cations 8, but the site-selectivity is total and seleno-functionalized allylic alcohols 9 are exclusively formed. Probably, the formation of intermediates 9 is also the (Z)/(E)-stereodetermining step. Oxidation of the hydroxyl group at intermediates 9 provides final products 7 and releases pyridin-1-ium triflate and hydrogen fluoride. Alternatively, the hydroxy group of intermediates 9 may arise not from external water, but from the ester group of starting allenes 2 based on the proposal by Fu and Ma.4f To clarify the effective engagement of external water in the selenofunctionalization of ethoxycarbonyl-allenes, an 18O-labeling experiment was performed (Scheme 8). Hence, the reaction of allene 2g was carried out under otherwise optimal conditions, but in the presence of one equivalent of H218O with an isotope abundance of 97%. In the event, the resulting 4-oxo-3-(phenylselanyl)pent-2-enoate 7g was 18O-labeled (ca. 50%) as detected by mass spectrometry (see the ESI‡), suggesting the involvement of adventitious water in this transformation.
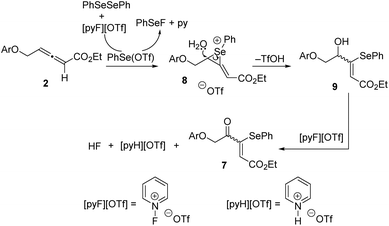 | ||
| Scheme 7 Proposed reaction pathway for the 1-fluoropyridinium triflate-assisted formation of α-selenoenones 7. | ||
To further expand the study of the 1,2-diene skeleton under these metal-free oxidative selenofunctionalization conditions, differently functionalized allenes 10–13 were surveyed (Scheme 9). Interestingly, allenamides 10a and 10b reacted smoothly to furnish the appropriate α-selenoenals 14a and 14b in reasonable yields with good Z-stereoselectivity. The behaviour of gem-disubstituted allenes 11 and 12 was less rewarding. The added steric bulk resulted in a complex reaction mixture for diphenyl allene 11, while allene 12 was suitable for this transformation. Surprisingly, the reaction of 1-methoxy-4-(1-methoxy-2-methylbuta-2,3-dien-1-yl)benzene 12 resulted in a separable mixture of selenoenal 15 and non-selenated enone 16. By contrast, allenone 13 failed to provide the corresponding α-selenoenone, giving instead selenated furan 17.
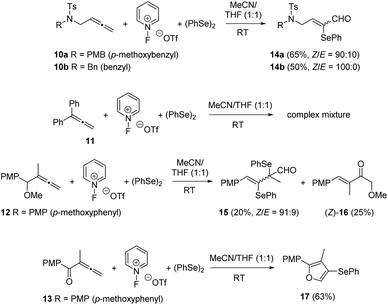 | ||
| Scheme 9 Reactivity of functionalized allenes 10–13 under oxidative selenofunctionalization conditions. | ||
Conclusions
In conclusion, we present the controlled preparation of two types of α-seleno-α,β-unsaturated carbonyls, namely, α-selenoenals and α-selenoenones, directly from allenes through metal-free oxidative selenofunctionalization reactions by a subtle variation of the substituents at the allene end. Consequently, the effect of the presence of an ethoxycarbonyl moiety at the allene end on the outcome of the process has been established. The decisive role of the organoselenium and 1-fluoropyridinium reagents has also been disclosed.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support for this work from MINECO and FEDER (projects CTQ2015-65060-C2-1-P and CTQ2015-65060-C2-2-P) is gratefully acknowledged. M. T.-P. thanks CAM and FEDER (YEI) for a predoctoral contract.Notes and references
- For a thematic issue on allene chemistry, see: (a) Progress in Allene Chemistry, ed. B. Alcaide and P. Almendros, Chem. Soc. Rev., 2014, 43, 2879–3205 (issue 9, themed collections). For representative reviews, see: ; (b) B. Yang, Y. Qiu and J.-E. Backvall, Acc. Chem. Res., 2018, 51, 1520 CrossRef CAS PubMed; (c) H.-U. Reissig and R. Zimmer, Synthesis, 2017, 49, 3291 CrossRef CAS; (d) A. Lledó, A. Pla-Quintana and A. Roglans, Chem. Soc. Rev., 2016, 45, 2010 RSC; (e) J. M. Alonso, M. T. Quirós and M. P. Muñoz, Org. Chem. Front., 2016, 3, 1186 RSC; (f) W. Yang and A. S. K. Hashmi, Chem. Soc. Rev., 2014, 43, 2941 RSC; (g) S. Kitagaki, F. Inagaki and C. Mukai, Chem. Soc. Rev., 2014, 43, 2956 RSC; (h) B. Alcaide and P. Almendros, Acc. Chem. Res., 2014, 47, 939 CrossRef CAS PubMed; (i) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074 CrossRef CAS PubMed.
- (a) K. N. Sands, T. A. Tuck and T. G. Back, Chem. – Eur. J., 2018, 24, 971 Search PubMed; (b) J. Kil, E. Lobarinas, C. Spankovich, S. K. Griffiths, P. J. Antonelli, E. D. Lynch and C. G. Le Prell, Lancet, 2017, 390, 969 CrossRef CAS PubMed; (c) E. A. Wilhelm, C. R. Jesse, C. F. Bortoletto, C. W. Nogueira and L. Savegnago, Pharmacol., Biochem. Behav., 2009, 93, 419 CrossRef CAS PubMed; (d) R. Asaf, S. Blum, R. Miller-Lotan and A. P. Levy, Lett. Drug Des. Discovery, 2007, 4, 160 CrossRef CAS; (e) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS PubMed; (f) M. Soriano-García, Curr. Med. Chem., 2004, 11, 1657 CrossRef.
- S. Shabaan, L. A. Ba, M. Abbas, T. Burkholz, A. Denkert, A. Gohr, L. A. Wessjohann, F. Sasse, W. Weber and C. Jacob, Chem. Commun., 2009, 4702 RSC.
- (a) B. Alcaide, P. Almendros, A. Luna, G. Gómez-Campillos and M. R. Torres, J. Org. Chem., 2012, 77, 3549 CrossRef CAS PubMed; (b) G. He, Y. Yu, C. Fu and S. Ma, Eur. J. Org. Chem., 2010, 101 CrossRef CAS; (c) S. Ma, Pure Appl. Chem., 2007, 79, 261 CAS; (d) S. Ma, Acc. Chem. Res., 2009, 42, 1679 CrossRef CAS PubMed; (e) G. He, H. Guo, R. Qian, Y. Guo, C. Fu and S. Ma, Tetrahedron, 2009, 65, 4877 CrossRef CAS; (f) G. Chen, C. Fu and S. Ma, J. Org. Chem., 2006, 71, 9877 CrossRef CAS PubMed; (g) G. Chen, C. Fu and S. Ma, Tetrahedron, 2006, 62, 4444 CrossRef CAS; (h) A. Ogawa, R. Obayashi, M. Doi, N. Sonoda and T. Hirao, J. Org. Chem., 1998, 63, 4277 CrossRef CAS.
- L. Liao, R. Guo and X. Zhao, Angew. Chem., Int. Ed., 2017, 56, 3201 CrossRef CAS PubMed.
- The α,β-unsaturated carbonyl moiety is a very useful building block in organic synthesis because it has led to many synthetically useful transformations. For selected reviews, see: (a) V. Marcos and J. Alemán, Chem. Soc. Rev., 2016, 45, 6812 RSC; (b) H. Pellissier, Tetrahedron, 2012, 68, 2197 CrossRef CAS; (c) A. Moyano and R. Ríos, Chem. Rev., 2011, 111, 4703 CrossRef CAS PubMed; (d) T. Jerphagnon, M. G. Pizzuti, A. J. Minnaard and B. L. Feringa, Chem. Soc. Rev., 2009, 38, 1039 RSC; (e) A. Alexakis, J. E. Bäckvall, N. Krause, O. Pámies and M. Diéguez, Chem. Rev., 2008, 108, 2796 CrossRef CAS PubMed; (f) I. Escher and F. Glorius, in Science of Synthesis, Vol. 25, ed. R. Brückner and E. Schaumann, Georg Thieme Verlag, Stuttgart, 2006, pp. 733–777 RSC. For a selected recent report on the usefulness of α-selenoenones, see: T. Kano, H. Maruyama, C. Homma and K. Maruoka, Chem. Commun., 2018, 54, 176 RSC.
- For selected reviews, see: (a) R. Guo, L. Liao and X. Zhao, Molecules, 2017, 22, 835 CrossRef PubMed; (b) A. S. Kiselyov, Chem. Soc. Rev., 2005, 34, 1031 RSC.
- P. Crabbé, H. Fillion, D. André and J. L. Luche, J. Chem. Soc., Chem. Commun., 1979, 860 Search PubMed.
- V. R. Sabbasani, P. Mamidipalli, H. Lu, Y. Xia and D. Lee, Org. Lett., 2013, 7, 1552 CrossRef PubMed.
- The (Z)-stereochemistry of α-selenoenals 3 and α-selenoenones 7 was unambiguously determined by the NOESY analysis of adducts 3e and 7h (see the ESI‡ for details).
- The metal-catalyzed hydroformylation reaction of allenes yields β,γ-unsaturated aldehydes: (a) J. Eshon, C. R. Landis and J. M. Schomaker, J. Org. Chem., 2017, 82, 9270 CrossRef CAS PubMed; (b) A. Koepfer and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 6913 CrossRef CAS PubMed; (c) C. F. Huo, Y. W. Li, M. Beller and H. Jiao, Chem. – Eur. J., 2005, 11, 889 CrossRef CAS PubMed.
- HPLC grade solvents were used: MeCN (99.9% purity; 0.03% water) and THF (99.9% purity; 0.02% water).
- For the rhodium-catalyzed preparation of β,γ-unsaturated carbonyl compounds from allenes and malononitrile, see: (a) C. P. Grugel and B. Breit, Chem. – Eur. J., 2018, 24, 15223 CrossRef CAS PubMed. For the silver-catalyzed preparation of α-trifluoromethylated enones from allenes and Langlois's salt, see: (b) M. Brochetta, T. Borsari, A. Gandini, S. Porey, A. Deb, E. Casali, A. Chakraborty, G. Zanoni and D. Maiti, Chem. – Eur. J., 2019, 25, 750 CrossRef CAS PubMed.
Footnotes |
| † In memory of Prof. Ángel Vidal. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, compound characterization data, and copies of NMR spectra for all new compounds. See DOI: 10.1039/c9qo00561g |
| This journal is © the Partner Organisations 2019 |