Synthesis of a dimer of the repeating unit of type Ia group B Streptococcus extracellular capsular polysaccharide and immunological evaluations of related protein conjugates†
Abstract
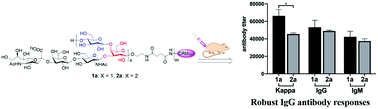
Type Ia group B Streptococcus (GBS) is one of the major causes of fatal infections in neonates. Its extracellular capsular polysaccharide (CPS) is a useful target for the development of anti-type Ia GBS vaccines. To explore the structure–activity relationships of type Ia GBS CPS and design more effective vaccines, a dimer of the branched pentasaccharide repeating unit of this CPS was synthesized by a highly convergent strategy highlighted by constructing the key intermediate via one-pot iterative glycosylation and imposing two side chains in one step via dual glycosylation. This represented the first total synthesis of a dimer of the repeating unit of any GBS CPS reported so far and the strategy should be applicable to higher oligomers of this repeating unit. The synthetic dimer and its monomeric analog were coupled with CRM197 carrier protein to generate conjugates that were evaluated in mice. Immunological results revealed that both carbohydrate antigens could induce robust total and IgG antibody responses and the elicited antibodies were cross-reactive with both carbohydrate antigens. It was concluded that both the monomeric and the dimeric repeating units may be employed as haptens for anti-type Ia GBS vaccine development.


 Please wait while we load your content...
Please wait while we load your content...