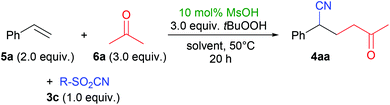Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Radical addition of ketones and cyanide to olefins via acid catalyzed formation of intermediate alkenyl peroxides†
Wen
Shao‡
a,
Marcel
Lux
a,
Martin
Breugst
 b and
Martin
Klussmann
b and
Martin
Klussmann
 *a
*a
aMax Planck Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 2, 45470 Mülheim an der Ruhr, Germany. E-mail: klussmann@mpi-muelheim.mpg.de
bUniversität zu Köln, Department für Chemie, Greinstraße 4, 50939 Köln, Germany
First published on 18th April 2019
Abstract
A Brønsted acid catalyzed method was developed for the synthesis of γ-cyanoketones from sulfonyl cyanides, olefins and ketones. The reaction is believed to proceed via intermediate formation of alkenyl peroxides by condensation of ketones with tert-butylhydroperoxide. These unstable compounds decompose by homolytic O–O bond cleavage, generating ketone-derived radicals which add to the olefins and generate the final products after reaction with the sulfonyl cyanide, thereby forming two new C–C bonds. A range of different ketones and olefins can be used, including steroidal ketones and simple alkyl olefins. The products can be further transformed to substituted lactones and piperidines, including a tetracyclic one. This reaction can thus be utilized to gain access to complex molecules from simple starting materials in only a few synthetic steps.
Introduction
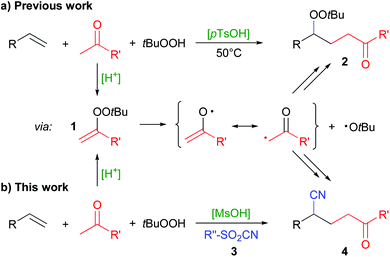
Radical addition reactions enable the double functionalization of olefins with heteroatom- and carbon-residues, thus much effort is put into their development.1 We have recently developed a method for the acid-catalyzed generation of radicals from ketones and hydroperoxides via the intermediate formation of alkenyl peroxides 1 (Scheme 1a).2 Alkenyl peroxides are highly unstable and can generally not be isolated at ambient temperature.3 Using this strategy, simple ketones can be utilized in radical reactions, for example towards the synthesis of γ-peroxyketones 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a and heterocycles.4
2a and heterocycles.4
 | ||
| Scheme 1 Radical addition reactions via intermediate alkenyl peroxides (1) formed by acid catalysis. | ||
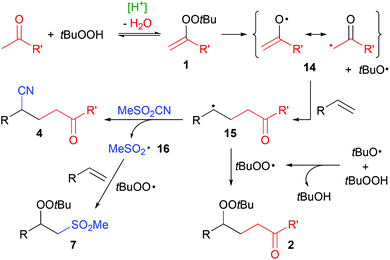
The generation of radicals in the α-position of ketones can be achieved by alternative means,5 but often it is only effective with 1,3-dicarbonyl compounds,6 at temperatures above 100 °C,7 with α-bromo-ketones as substrates8 and larger amounts of metal reagents,9 respectively. Their formation via alkenyl peroxides (1) by condensation with hydroperoxides, on the other hand, is particularly effective with plain ketones and only requires simple acids as catalysts. We were thus interested to extend the radical addition of ketones via this mechanism to the twofold addition of carbon-radicals instead of the carbo-peroxidation as in the synthesis of 2. Here, we report our results of the addition of ketones and cyanide to olefins (Scheme 1b).
We tested a variety of additional reagents to introduce a carbon residue instead of the peroxide group, but the peroxyketones 2 were always formed as major products. Overriding this preference was finally possible by employing sulfonyl cyanides 3 which led to formation of cyanoketones 4. Sulfonyl cyanides are well-known reagents for the transfer of a cyano group to carbon-radicals.10 For example, the Landais group used para-toluenesulfonic cyanide (pTsCN, 3a) in the radical addition of ester, thioester or amide residues to olefins, requiring the use of stoichiometric organometallic reagents and prefunctionalized substrates, respectively.10g–i Radical trifluoromethylation with hypervalent iodine reagents together with cyanation has been reported by several groups.11 The groups of Liu and Zhu reported an intramolecular CN-transfer after radical addition, requiring special olefin substrates.12 The strategy of an intramolecular CN-transfer had previously been employed by the groups of Kalvoda, Watt and Nikishin to synthesize γ-cyanoketones.13 However, the yield in those reactions was typically rather low. Zhu et al. also described such products via a radical ring-opening reaction of cyclobutanols in the presence of pTsCN.10f
γ-Cyanoketones can also be prepared in a single step by conjugate addition reactions of ketones to vinyl nitriles and of nitriles to enones, respectively, generally requiring strong bases or complex catalysts.14 Applying chiral catalysts, this can allow the stereoselective synthesis of the products, but at the same time requiring nitriles with electron-withdrawing groups in the α-position.15
The method we have developed could be complementary to those known cases. It uses readily available ketones and olefins and can be catalyzed by simple Brønsted acids. Directly adding ketone and cyanide radicals to an olefin could also allow the synthesis of γ-cyanoketones not easily accessible by the aforementioned methods.
Results and discussion
During the optimization of the method, we screened many different conditions, e.g. solvents, acids, cyanide reagents and concentrations. Full details can be found in the ESI,† and exemplary results are shown in Table 1. The desired product 4aa was formed in good yields from pTsCN (3a), styrene (5a) and acetone (6a) in polar aprotic solvents (entries 1–3). Slightly better results were achieved when using ethyl- or methylsulfonyl cyanides 3b and 3c (entries 4 and 5),§ which can easily be synthesized by oxidation of the thiocyanates.10h We achieved the best results with 3c in dichloromethane (entry 5).| Entry | R (3) | Solvent | Yieldb/% |
|---|---|---|---|
| a Screening reactions were typically performed with 0.5 mmol 5a, 1.25 mmol 6a, 0.25 mmol 3, 1.0 mmol tBuOOH and 0.025 mmol acid in 0.2–1.0 ml solvent under an argon atmosphere at 50 °C. b Determined by 1H-NMR. c Isolated yield, reaction at a scale of 0.5 mmol 3, 1.5 mmol 6a, 1.5 mmol tBuOOH and 1.0 ml of CH2Cl2. | |||
| 1 | pTol (3a) | EtOAc | 60 |
| 2 | pTol (3a) | CHCl3 | 48 |
| 3 | pTol (3a) | CH2Cl2 | 61 |
| 4 | Et (3b) | CH2Cl2 | 64 |
| 5 | Me (3c) | CH2Cl2 | 69 (63)c |
In those reactions, two byproducts could be characterized (Fig. 1). Peroxyketone 2aa – the product formed in the absence of sulfonyl cyanides2a – was found in low amounts of up to 10% only. Peroxy-sulfone167ac was formed in comparable, but generally lower yields than cyanoketone 4aa. In order to achieve good yields of the desired products, the olefin therefore has to be used in excess. Generally, sulfones 7 can easily be separated from nitriles 4 by column chromatography.
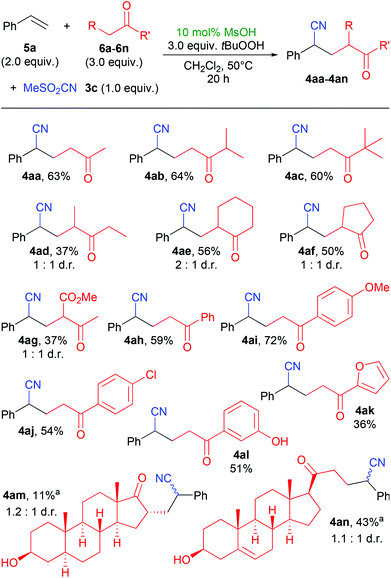
Using the conditions as of Table 1, entry 5, we investigated this method in the reactions of different ketones with styrene (Scheme 2). Simple open-chain and cyclic alkyl ketones gave the expected products 4aa–4ag. With methyl isopropyl ketone, the new bond was exclusively formed at the methyl group (4ab). Methyl aryl ketones afforded products 4ah–4al in similar yields. The successful utilization of phenolic ketone 6l in the synthesis of 4al is interesting, as many phenols are known as radical inhibitors. Two steroids, epiandrosterone 6m and pregnenolone 6n could be used as ketone component, giving the products 4am and 4an. The presence of a free hydroxyl group and an olefin in the ketone 6n did not hamper the yield significantly, as 4an was formed in a relatively good yield of 43%.
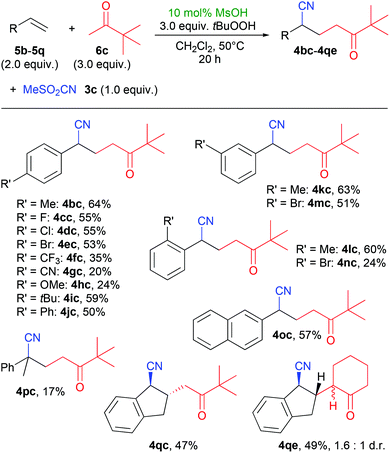
With pinacolone (6c) as a model ketone, we also tested the reaction with a variety of different aryl olefins (Scheme 3). A range of products 4bc–4nc with different aryl-substituents were formed in mostly good yields above 50%, with the exception of the strongly electron-withdrawing and -donating substituents CF3, CN and OMe (4fc–4hc). A methyl substituent in the ortho-position also gave a good yield of 60% of 4lc, but the ortho-bromide product 4nc gave a lower yield. Using indene (5q) as olefin gave the products 4qc and 4qe (using cyclohexanone) in good yields, which are remarkable for their high trans-selectivity. For 4qc, a diastereomeric ratio of >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was determined in the crude reaction mixture, but after purification, we received the pure trans-product. In the case of 4qe, two diastereomers were isolated which differ in the configuration at the α-position of the cyclohexanone residue.
1 was determined in the crude reaction mixture, but after purification, we received the pure trans-product. In the case of 4qe, two diastereomers were isolated which differ in the configuration at the α-position of the cyclohexanone residue.
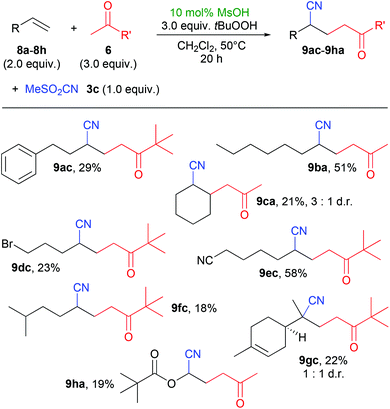
We also investigated the reaction between acetone or pinacolone and non-aryl olefins which on average gave lower yields than with styrene derivatives (Scheme 4). This is possibly due to the nature of the ketone-derived radicals, as this had also been observed during the synthesis of γ-peroxyketones.2a Noteworthy exceptions were the use of 1-octene (8b) and 6-cyano-1-hexene (8e), providing yields above 50%. A natural product, limonene (8g) could be used as olefin component, albeit with only a rather low yield of 22% of product 9gc. Interestingly, in the reactions with these olefins, the sulfonyl peroxide byproducts 7 were observed in traces only and could not be isolated.
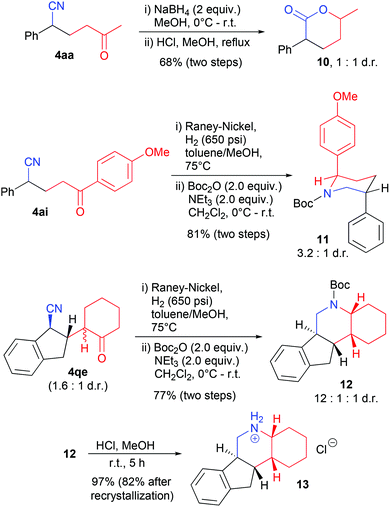
Cyanoketones have previously been utilized as valuable synthetic intermediates that can be converted to lactones and piperidines, respectively, by reduction and subsequent cyclization.14a,15c–e,17 We have also applied these strategies to selected products, without purification after the first step (Scheme 5). A substituted δ-valerolactone 10 was formed from product 4aa in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers. Reduction of the nitrile group by hydrogenation in the presence of RANEY® Nickel and subsequent protection with a Boc-group gave piperidines 11 and 12 from cyanoketones 4ai and 4qe, respectively.
1 mixture of diastereomers. Reduction of the nitrile group by hydrogenation in the presence of RANEY® Nickel and subsequent protection with a Boc-group gave piperidines 11 and 12 from cyanoketones 4ai and 4qe, respectively.
Interestingly, the major diastereomer of 11 was found to obtain a solution conformation with both aryl groups in axial position, as shown in Scheme 5 (determined by NMR coupling constants and an NOESY experiment, see the ESI† for details). This at first surprising result was supported by DFT calculations:18 this conformation was indeed found to be more stable than the one with both aryl groups in equatorial position by 3.3 kJ mol−1 and also more stable than alternative boat conformations. This results presumably from the avoidance of steric repulsion between the p-methoxyphenyl and the Boc group19 and from a favorable orbital interaction between the nitrogen lone pair and the σ*-bond of the C–aryl bond (see the ESI† for further details).
Tetracylic piperidine 12 was formed with a high preference for one diastereomer, as depicted in Scheme 5. The structure of the major one was confirmed by NMR spectroscopy and X-ray crystallography of the corresponding ammonium salt 13, which was isolated diastereomerically pure after recrystallization (see the ESI† for further details).
In accordance with our earlier mechanistic studies of alkenyl peroxide formation2b and based on the observation of byproducts 2 and 7, a radical mechanism appears reasonable (Scheme 6). Alkenyl peroxides 1 are formed by acid-catalyzed condensation of ketones and tBuOOH. Rapid homolytic O–O cleavage generates a tert-butyloxyl radical and resonance-stabilized radical 14, which adds to an olefin, forming the intermediate C-radical 15. Reaction with MeSO2CN10c generates the cyanoketones 4 and a sulfonyl radical 16. Rapid H-atom transfer from tBuOOH to the oxyl radical generates the tert-butyl peroxyl radical,20 which can attack 15, forming peroxyketone 2 as minor byproduct. Major byproduct 7 is formed by subsequent attack of a sulfonyl and peroxyl radical on an alkene.16,21
Conclusions
In summary, we have developed a Brønsted-acid catalyzed synthetic method for the radical addition of ketones and cyanide to olefins, achieving the formation of two new C–C bonds. A variety of ketones can be added to styrene derivatives but also to various alkyl olefins, giving a range of structurally diverse γ-cyanoketones. Selected products were further converted to a lactone and two piperidines, including the case of a tetracyclic piperidine that was thus synthesized in three steps only from simple starting materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the DFG (KL 2221/7-1 and KL 2221/4-2, Heisenberg scholarship to M.K.) and the Max-Planck-Institut fuer Kohlenforschung for funding. We are grateful to the Regional Computing Center of the University of Cologne for providing computing time of the DFG-funded High Performance Computing (HPC) System CHEOPS as well as for their support. Open Access funding provided by the Max Planck Society.Notes and references
- (a) X.-W. Lan, N.-X. Wang and Y. Xing, Eur. J. Org. Chem., 2017, 5821 CrossRef CAS; (b) J. M. Muñoz-Molina, T. R. Belderrain and P. J. Pérez, Eur. J. Inorg. Chem., 2011, 2011, 3155 CrossRef; (c) H. Fischer and L. Radom, Angew. Chem., Int. Ed., 2001, 40, 1340 CrossRef CAS; (d) B. Giese, Angew. Chem., Int. Ed. Engl., 1983, 22, 753 CrossRef.
- (a) B. Schweitzer-Chaput, J. Demaerel, H. Engler and M. Klussmann, Angew. Chem., Int. Ed., 2014, 53, 8737 CrossRef CAS PubMed; (b) B. Schweitzer-Chaput, T. Kurtén and M. Klussmann, Angew. Chem., Int. Ed., 2015, 54, 11848 CrossRef CAS PubMed.
- M. Klussmann, Chem. – Eur. J., 2018, 24, 4480 CrossRef CAS PubMed.
- (a) E. Boess, S. Karanestora, A.-E. Bosnidou, B. Schweitzer-Chaput, M. Hasenbeck and M. Klussmann, Synlett, 2015, 26, 1973 CrossRef CAS; (b) X.-F. Xia, S.-L. Zhu, M. Zeng, Z. Gu, H. Wang and W. Li, Tetrahedron, 2015, 71, 6099 CrossRef CAS; (c) S.-L. Zhu, P.-X. Zhou and X.-F. Xia, RSC Adv., 2016, 6, 63325 RSC; (d) C.-S. Wang, T. Roisnel, P. H. Dixneuf and J.-F. Soulé, Adv. Synth. Catal., 2019, 361, 445 CAS.
- (a) M. S. Kharasch, J. Kuderna and W. Nudenberg, J. Org. Chem., 1953, 18, 1225 CrossRef CAS; (b) G. I. Nikishin, G. V. Somov and A. D. Petrov, Russ. Chem. Bull., 1961, 10, 1924 CrossRef; (c) T. Iwahama, S. Sakaguchi and Y. Ishii, Chem. Commun., 2000, 36, 2317 RSC; (d) H. Wang, L.-N. Guo and X.-H. Duan, Chem. Commun., 2013, 49, 10370 RSC; (e) L. Zhu, H. Chen, Z. Wang and C. Li, Org. Chem. Front., 2014, 1, 1299 RSC; (f) X.-W. Lan, N.-X. Wang, W. Zhang, J.-L. Wen, C.-B. Bai, Y. Xing and Y.-H. Li, Org. Lett., 2015, 17, 4460 CrossRef CAS PubMed; (g) Y. Tian, C. Sun, R. X. Tan and Z.-Q. Liu, Green Chem., 2018, 20, 588 RSC.
- (a) B. B. Snider, Chem. Rev., 1996, 96, 339 CrossRef CAS PubMed; (b) B. B. Snider, Tetrahedron, 2009, 65, 10738 CrossRef CAS PubMed; (c) M. Rössle, T. Werner, W. Frey and J. Christoffers, Eur. J. Org. Chem., 2005, 5031 CrossRef; (d) X. Zheng, S. Lu and Z. Li, Org. Lett., 2013, 15, 5432 CrossRef CAS PubMed.
- (a) C. Pan, Z. Yang, D. Gao and J.-T. Yu, Org. Biomol. Chem., 2018, 16, 6035 RSC; (b) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-J. Ji, Chem. – Eur. J., 2014, 20, 17198 CrossRef CAS PubMed.
- (a) F. Zhang, P. Du, J. Chen, H. Wang, Q. Luo and X. Wan, Org. Lett., 2014, 16, 1932 CrossRef PubMed; (b) S. N. Gockel, T. L. Buchanan and K. L. Hull, J. Am. Chem. Soc., 2018, 140, 58 CrossRef CAS PubMed.
- (a) E.-A. I. Heiba and R. M. Dessau, J. Am. Chem. Soc., 1971, 93, 524 CrossRef; (b) U. Linker, B. Kersten and T. Linker, Tetrahedron, 1995, 51, 9917 CrossRef; (c) J. Xie and Z.-Z. Huang, Chem. Commun., 2010, 46, 1947 RSC; (d) Y. Li, J.-Q. Shang, X.-X. Wang, W.-J. Xia, T. Yang, Y. Xin and Y.-M. Li, Org. Lett., 2019, 21, 2227 CrossRef CAS PubMed.
- (a) R. G. Pews and T. E. Evans, J. Chem. Soc. D, 1971, 1397 RSC; (b) J.-M. Fang and M.-Y. Chen, Tetrahedron Lett., 1987, 28, 2853 CrossRef CAS; (c) D. H. R. Barton, J. C. Jaszberenyi and E. A. Theodorakis, Tetrahedron, 1992, 48, 2613 CrossRef; (d) A.-P. Schaffner, V. Darmency and P. Renaud, Angew. Chem., Int. Ed., 2006, 45, 5847 CrossRef CAS PubMed; (e) S. Kamijo, T. Hoshikawa and M. Inoue, Org. Lett., 2011, 13, 5928 CrossRef CAS PubMed; (f) R. Ren, Z. Wu, Y. Xu and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 2866 CrossRef CAS PubMed; (g) N. S. Dange, F. Robert and Y. Landais, Org. Lett., 2016, 18, 6156 CrossRef PubMed; (h) H. Hassan, V. Pirenne, M. Wissing, C. Khiar, A. Hussain, F. Robert and Y. Landais, Chem. – Eur. J., 2017, 23, 4651 CrossRef PubMed; (i) R. Hara, C. Khiar, N. S. Dange, P. Bouillac, F. Robert and Y. Landais, Eur. J. Org. Chem., 2018, 4058 CrossRef CAS.
- (a) N. O. Ilchenko, P. G. Janson and K. J. Szabó, J. Org. Chem., 2013, 78, 11087 CrossRef CAS PubMed; (b) Z. Liang, F. Wang, P. Chen and G. Liu, J. Fluorine Chem., 2014, 167, 55 CrossRef CAS; (c) Y.-T. He, L.-H. Li, Y.-F. Yang, Z.-Z. Zhou, H.-L. Hua, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2014, 16, 270 CrossRef CAS PubMed; (d) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Org. Lett., 2014, 16, 1240 CrossRef CAS PubMed.
- (a) N. Wang, L. Li, Z.-L. Li, N.-Y. Yang, Z. Guo, H.-X. Zhang and X.-Y. Liu, Org. Lett., 2016, 18, 6026 CrossRef CAS PubMed; (b) R. Ren, Z. Wu, L. Huan and C. Zhu, Adv. Synth. Catal., 2017, 359, 3052 CrossRef CAS; (c) for a related strategy, see also: M. Wang, L. Huan and C. Zhu, Org. Lett., 2019, 21, 821 CrossRef CAS PubMed.
- (a) C. Meystre, K. Heusler, J. Kalvoda, P. Wieland, G. Anner and A. Wettstein, Experientia, 1961, 17, 475 CrossRef CAS; (b) J. Kalvoda, Helv. Chim. Acta, 1968, 51, 267 CrossRef CAS PubMed; (c) R. W. Freerksen, W. E. Pabst, M. L. Raggio, S. A. Sherman, R. R. Wroble and D. S. Watt, J. Am. Chem. Soc., 1977, 99, 1536 CrossRef CAS PubMed; (d) Y. N. Ogibin, D. S. Velibekova, É. I. Troyanskii and G. I. Nikishin, Russ. Chem. Bull., 1981, 30, 475 CrossRef; (e) É. I. Troyanskii, V. V. Mizintsev, A. N. Molokanov, Y. N. Ogibin and G. I. Nikishin, Russ. Chem. Bull., 1986, 35, 671 CrossRef; (f) É. I. Troyanskii, V. V. Mizintsev, A. N. Molokanov, Y. N. Ogibin and G. I. Nikishin, Russ. Chem. Bull., 1986, 35, 2499 CrossRef.
- (a) H. Henecka, Chem. Ber., 1949, 82, 104 CrossRef CAS; (b) R. Longeray and J. Dreux, Bull. Soc. Chim. Fr., 1963, 2805 CAS; (c) S. Paganelli, A. Sehionato and C. Botteghi, Tetrahedron Lett., 1991, 32, 2807 CrossRef CAS; (d) M. Vogt, A. Nerush, M. A. Iron, G. Leitus, Y. Diskin-Posner, L. J. W. Shimon, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2013, 135, 17004 CrossRef CAS PubMed; (e) A. Nerush, M. Vogt, U. Gellrich, G. Leitus, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2016, 138, 6985 CrossRef CAS PubMed.
- For reviews, see: (a) M. D. Díaz-de-Villegas, J. A. Gálvez, R. Badorrey and P. López-Ram-de-Víu, Adv. Synth. Catal., 2014, 356, 3261 CrossRef; (b) S. Jautze and R. Peters, Synthesis, 2010, 365 CAS. For selected examples, see: (c) M. S. Taylor, D. N. Zalatan, A. M. Lerchner and E. N. Jacobsen, J. Am. Chem. Soc., 2005, 127, 1313 CrossRef PubMed; (d) T.-Y. Liu, R. Li, Q. Chai, J. Long, B.-J. Li, Y. Wu, L.-S. Ding and Y.-C. Chen, Chem. – Eur. J., 2007, 13, 319 CrossRef CAS PubMed; (e) Y. Kawato, N. Takahashi, N. Kumagai and M. Shibasaki, Org. Lett., 2010, 12, 1484 CrossRef CAS PubMed.
- For an alternative synthesis of peroxy sulfones, see: R. Xu and Z. Li, Tetrahedron Lett., 2018, 59, 3942 CrossRef CAS.
- R. Longeray and J. Dreux, Compt. Rend., 1961, 252, 754 CAS.
- Calculated at the DLPNO-CCSD(T)/def2-TZVPP/CPCM(CHCl3)//TPSS-D3/6-311+G(d,p)/CPCM level of theory.
- For a review about conformational effects of this kind (1,3 axial strain), see: (a) F. Johnson, Chem. Rev., 1968, 68, 375 CrossRef CAS. For selected related cases, see: (b) F. Johnson, Chem. Rev., 1968, 68, 375 CrossRef CAS; (c) J. Quick, C. Mondello, M. Humora and T. Brennan, J. Org. Chem., 1978, 43, 2705 CrossRef CAS.
- (a) H. Paul, R. D. Small and J. C. Scaiano, J. Am. Chem. Soc., 1978, 100, 4520 CrossRef CAS; (b) D. V. Avila, K. U. Ingold, J. Lusztyk, W. H. Green and D. R. Procopio, J. Am. Chem. Soc., 1995, 117, 2929 CrossRef CAS.
- For a mechanistic study on the addition of sulfonyl radicals, see: C. Chatgilialoglu, O. Mozziconacci, M. Tamba, K. Bobrowski, G. Kciuk, M. P. Bertrand, S. Gastaldi and V. I. Timokhin, J. Phys. Chem. A, 2012, 116, 7623 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental and computational results. CCDC 1878410. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qo00447e |
| ‡ Present address: Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland. |
| § The superiority of MsCN (3c) over pTsCN (3a) had already been noted before, see ref. 10c. |
| This journal is © the Partner Organisations 2019 |