Reactivity of epoxy-ynamides with metal halides: nucleophile (Br/Cl/OH)-assisted tandem intramolecular 5-exo-dig or 6-endo-dig cyclisation and AgF2-promoted oxidation†
Abstract
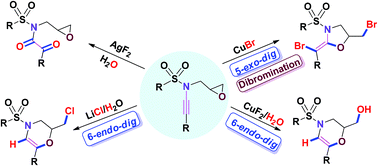
Several metal halides react with epoxy-ynamides. Thus, CuBr-mediated concomitant bromination/5-exo-dig cyclisation of epoxy-ynamides affords functionalised 1,3-oxazolidines. In contrast, functionalised 1,4-oxazines are obtained via 6-endo-dig cyclisation of epoxy-ynamides using LiCl or CuF2/H2O. In addition, CuBr is effective for dibromination of functionalised ynamides leading to α,β-dibromo-enamides, with the (E) isomer predominating. Formation of epoxy-1,2-dioxo-amides by oxidation of epoxy-ynamides using AgF2 as the oxidising agent is also highlighted.


 Please wait while we load your content...
Please wait while we load your content...