Application of dialkyl azodicarboxylate frameworks featuring multi-functional properties
Abstract
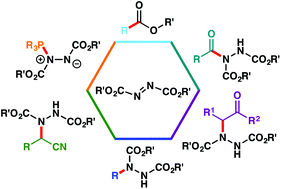
Azo-disubstituted derivatives with electron-withdrawing groups, such as acyl, alkoxycarbonyl and nitrile, are widely used as reagents in organic synthesis. Dialkyl azodicarboxylates are the most readily available reagents among them. This review summarizes the utility of dialkyl azodicarboxylates as versatile reagents and essential building blocks for the synthesis of complex molecules. Applications of dialkyl azodicarboxylates in the Mitsunobu reaction, as oxidants in the oxidation of aldehydes, hydrazines, alcohols, hydroxylamines and thiols, and in photocatalytic C–C bond cleavage and C–H bond amination reactions are discussed. In synthetic chemistry, a large number of synthetic procedures use azodicarboxylates as electrophiles. The discussion is also extended toward the enantioselective α-amination of carbonyl, cyanoacetate and heterocycle derivatives. In addition, mechanistic insights are also briefly reviewed.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...