Water-compatible fluorescent [2]rotaxanes for Au3+ detection and bioimaging†
Sing-Ming
Chan
a,
Fung-Kit
Tang
a,
Chak-Shing
Kwan
a,
Ching-Yau
Lam
a,
Sam C. K.
Hau
b and
Ken Cham-Fai
Leung
 *a
*a
aDepartment of Chemistry, Hong Kong Baptist University, Kowloon Tong, Kowloon, Hong Kong SAR, P. R. China. E-mail: cfleung@hkbu.edu.hk
bDepartment of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, P. R. China
First published on 10th September 2019
Abstract
In this study, the synthesis of [2]rotaxanes as fluorescent metal ion sensors has been demonstrated. [2]Rotaxanes, RA-H·PF6, RRA and RRB, undergo photoelectron transfer resulting in fluorescence quenching. Before the diimine (dynamic covalent bond) reduction on the macrocyclic ring, the dynamic [2]rotaxane RA-H·PF6 can be hydrolyzed and turned fluorescent by trivalent metal ions, giving fluorescence at λmax 424 nm. After reduction of the imines, the reduced [2]rotaxanes RRA and RRB are kinetically stable and highly selective to Au3+ binding among 27 metal ions in a water-compatible (50 vol%) solution with working fluorescence in the range of pH 4–10. 50-Fold and 1.2-fold fluorescence turn-on after addition of Au3+ has been observed for RRA and RRB, respectively. Metal interference on Au3+ detection is insignificant, and thereby the fluorescence intensity is linearly proportional to the concentration of Au3+ until excess. The solid-state crystal structure of RRA shows the mechanically interlocked structure (mechanical bond). The bioimaging experiment of RRB with HeLa cells demonstrates the potential application of these mechanically interlocked molecules for metal ion detection in aqueous media and biological systems.
Introduction
Simple organic molecules have been used as fluorescent metal ion sensors due to their high selectivity, sensitivity, simplicity, quick response and feasibility of both in vitro and in vivo testing.1–4 In particular, rotaxanes, mechanically interlocked molecules with unique topology,5,6 have been in the spotlight for ion sensing over the past few decades. Compared to conventional sensors, the interlocked system of rotaxanes can provide a dynamic and yet switchable cavity, thus allowing them to bind specific analytes selectively.7 The mechanical bond can also provide unusual coordination geometries and augmented redox activity as a potential ligand.8 It was first reported by Hiratani and co-workers as a selective Li+ fluorescent rotaxane sensor in CH2Cl2/MeCN solution (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v).9 Previously, Goldup and co-workers reported a [2]rotaxane fluorescent sensor for Zn2+ in MeCN/H2O solution (98
10, v/v).9 Previously, Goldup and co-workers reported a [2]rotaxane fluorescent sensor for Zn2+ in MeCN/H2O solution (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v).10 In another study performed by Beer and co-workers, they designed a rotaxane for sensing halide ions in up to 35% H2O in MeCN.11 Lin and co-workers also reported a [2]rotaxane for Fe3+ detection and bioimaging.12 However, most of the rotaxane sensors focus on alkali metal ions,9,13,14 divalent transition metal ions10,15 and anions.11,16–18 In the case of precious metals and other platinum group elements (Pt, Pd, Rh, Ir, Ru, and Os), the application of using rotaxanes as a metal ion sensor in aqueous media is relatively unexplored, and thereby the effect of other metal ions as interference is seldom mentioned.10 Since the detection of metal ions in aqueous media has become crucial in biological systems, the development of water-soluble rotaxane metal ion sensors has become highly desired.
2, v/v).10 In another study performed by Beer and co-workers, they designed a rotaxane for sensing halide ions in up to 35% H2O in MeCN.11 Lin and co-workers also reported a [2]rotaxane for Fe3+ detection and bioimaging.12 However, most of the rotaxane sensors focus on alkali metal ions,9,13,14 divalent transition metal ions10,15 and anions.11,16–18 In the case of precious metals and other platinum group elements (Pt, Pd, Rh, Ir, Ru, and Os), the application of using rotaxanes as a metal ion sensor in aqueous media is relatively unexplored, and thereby the effect of other metal ions as interference is seldom mentioned.10 Since the detection of metal ions in aqueous media has become crucial in biological systems, the development of water-soluble rotaxane metal ion sensors has become highly desired.
Gold, one of the precious metals, has been utilized in functional nanoparticles.19 In particular, its ion form, Au3+, has been applied in biological,20,21 catalysis,22,23 and other industrial applications.24 For instance, recent studies have shown that an Au(III) complex could be used as an effective anticancer drug due to its tumor growth inhibiting properties.21 Despite the advantages, excess Au3+ can damage the organs.25 For example, Au3+ can lead to inhibition of the oxygen consumption of liver and kidney slices.26 An Au3+ salt solution is over 90% toxic at a concentration of 200 μM.27 Thus, the detection of Au3+ has become crucial.
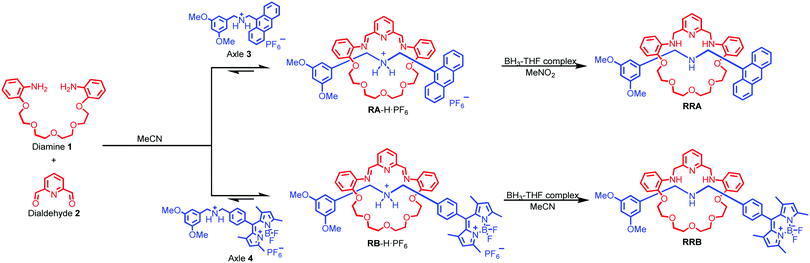
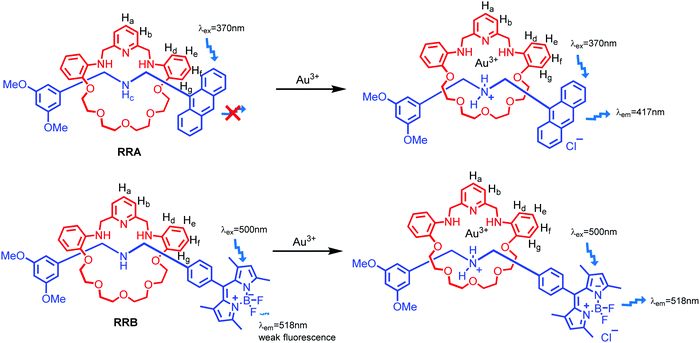
Herein, we report fluorescent [2]rotaxanes as selective metal ion sensors for detecting Au3+ and other trivalent metal ions in aqueous media by applying dynamic clipping on R2NH2+ for [2]rotaxane synthesis. It is part of dynamic covalent chemistry (DCC), which allows self-error checking and self-sorting to give the most thermodynamically stable rotaxane as the major product.28–36 The rotaxanes reported in this project were obtained in high yield, having pyridine/amine nitrogen and ethylene glycol oxygen donor atom moieties for selective metal ion binding. First, the fluorescent response of the as-synthesized RA-H·PF6 and RB-H·PF6 with the trivalent metal ions in 1% H2O in MeCN was investigated. To increase the selectivity, RA-H·PF6 and RB-H·PF6 were further reduced, and the fluorescence response shows much significant improvement for RRA and RRB on detection of Au3+ ions in MeCN/H2O solution. In view of potential bio-application of rotaxanes such as drug delivery,37–40 the cell viability of HeLa cells was performed to demonstrate the in vitro imaging of Au3+ with both RRA and RRB.
Results and discussion
As shown in Scheme 1, two dynamic rotaxanes, RA-H·PF6 and RB-H·PF6, were first synthesized for trivalent metal ion sensing. RA-H·PF6 was thermodynamically formed via a clipping strategy with equal molar concentrations of tetraethylene glycol bis(2-aminophenyl)ether (diamine 1), 2,6-pyridine dicarboxaldehyde (dialdehyde 2) and anthracene-based axle 3.41 On the other hand, RB-H·PF6 was synthesized with 1, 2, and BODIPY-based axle 4 by using the same clipping strategy. After the [2]rotaxane formation, the original fluorescence from the anthracene-based and BODIPY-based axles could be quenched, which may be caused by the electron transfer from the imine and pyridine moieties within the macrocyclic ring. The fluorescence could be restored when the imine moieties of RA-H·PF6 hydrolyze back to the aldehyde and amine groups in the presence of acid or water, which leads to the dissociation of the macrocycle and separate components. The breakdown will then inhibit the internal electron transfer and provide a fluorescence band with λmax at 424 nm.The fluorescence response of RA-H·PF6 towards cations Li+, NH4+, Na+, K+, Ag+, Cs+, Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Pd2+, Cd2+, Pt2+, Hg2+, Pb2+, Al3+, V3+, Cr3+, Fe3+, Ru3+, Rh3+, Ce3+, Ir3+ and Au3+ was first investigated in a MeCN/H2O (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
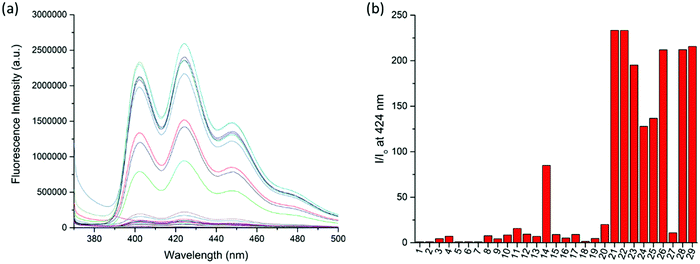
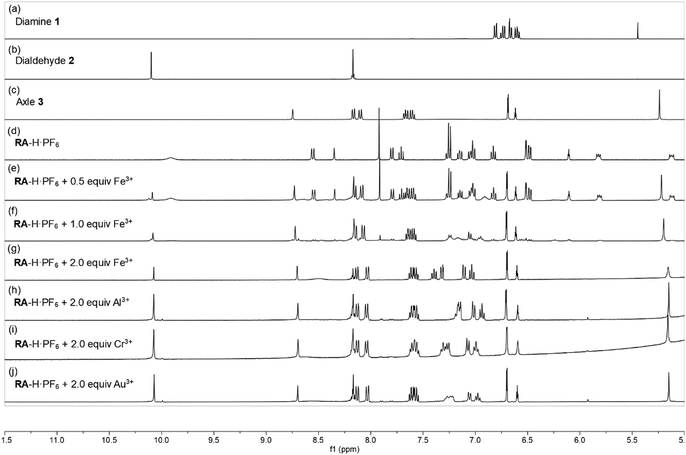
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) co-solvent system. As shown in Fig. 1, the fluorescence of RA-H·PF6 was turned on significantly by 5 equivalents of common trivalent metal ions like Al3+, V3+, Cr3+, Fe3+, Ru3+, Rh3+, Ir3+ and Au3+, along with moderate fluorescence turn-on by a divalent metal ion (Cu2+). These results indicate that dynamic [2]rotaxane RA-H·PF6 is relatively selective to reported trivalent metal ion binding. Al, V, Cr, and Fe are high abundance elements on Earth. Ru, Rh, Ir and Au are commonly used as catalysts for organic reactions. Since those trivalent metal ions are Lewis acids, they could induce acid hydrolysis of the imine moiety of RA-H·PF6 upon binding to the dynamic [2]rotaxane (Scheme 2). To study the mechanism, NMR spectroscopic titration experiments were conducted. The stacked 1H NMR spectra (Fig. 2) illustrate that the addition of trivalent metal ions could decrease the amount of imine protons of RA-H·PF6 while increasing the amount of aldehyde protons. This also supports that the hydrolyzed RA-H·PF6 could be separated into diamine, dialdehyde and axle components upon the addition of trivalent metal ions. Moreover, the axle 3 could restore its fluorescence because of the absence of PET.
1, v/v) co-solvent system. As shown in Fig. 1, the fluorescence of RA-H·PF6 was turned on significantly by 5 equivalents of common trivalent metal ions like Al3+, V3+, Cr3+, Fe3+, Ru3+, Rh3+, Ir3+ and Au3+, along with moderate fluorescence turn-on by a divalent metal ion (Cu2+). These results indicate that dynamic [2]rotaxane RA-H·PF6 is relatively selective to reported trivalent metal ion binding. Al, V, Cr, and Fe are high abundance elements on Earth. Ru, Rh, Ir and Au are commonly used as catalysts for organic reactions. Since those trivalent metal ions are Lewis acids, they could induce acid hydrolysis of the imine moiety of RA-H·PF6 upon binding to the dynamic [2]rotaxane (Scheme 2). To study the mechanism, NMR spectroscopic titration experiments were conducted. The stacked 1H NMR spectra (Fig. 2) illustrate that the addition of trivalent metal ions could decrease the amount of imine protons of RA-H·PF6 while increasing the amount of aldehyde protons. This also supports that the hydrolyzed RA-H·PF6 could be separated into diamine, dialdehyde and axle components upon the addition of trivalent metal ions. Moreover, the axle 3 could restore its fluorescence because of the absence of PET.
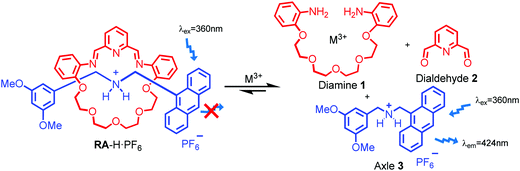 | ||
| Scheme 2 Plausible mechanism of switch on fluorescence of RA-H·PF6 to trivalent metal ions via rotaxane dissociation. | ||
With the aim to investigate another metal sensing mechanism without cleavage of the macrocyclic ring of dynamic [2]rotaxane, the imine moieties on the macrocycle of the dynamic [2]rotaxane RA-H·PF6 and RB-H·PF6 were then reduced by BH3–THF complex (Scheme 1), yielding diamine-based, kinetically stable [2]rotaxanes RRA and RRB. After the purification by column chromatography, RRA and RRB were obtained in 70% and 75% yield, respectively. The X-ray crystallography analysis of RRA single crystal shows the existence of the interlocking structure (mechanical bond) in the solid-state structure (Fig. 3), confirming successful diimine reduction to form the RRA.
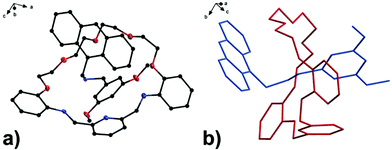 | ||
| Fig. 3 (a) Perspective view of solid-state structures of reduced [2]rotaxane RRA; (b) discriminative view of solid-state structures of reduced [2]rotaxane RRA. CCDC code: 1942423.† | ||
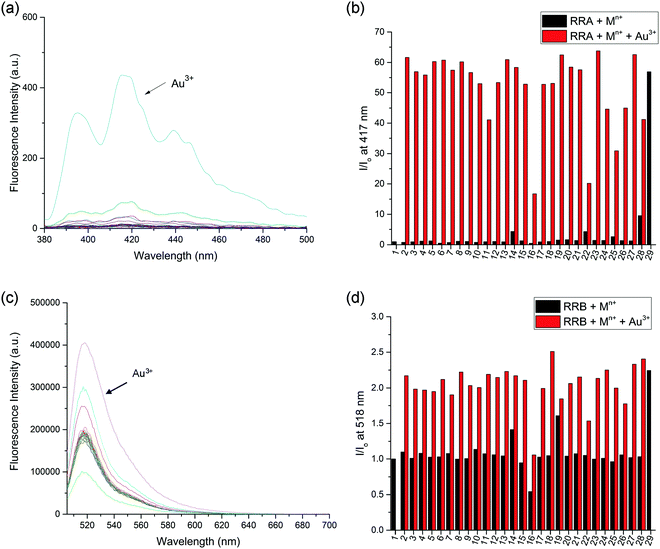
The selectivity of the reduced rotaxanes to cations was investigated. Since the solubility of RRA is relatively low at MeCN, THF was used to dissolve RRA. After the diimine reduction, the fluorescence response of RRA in THF/MeCN/H2O solution (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
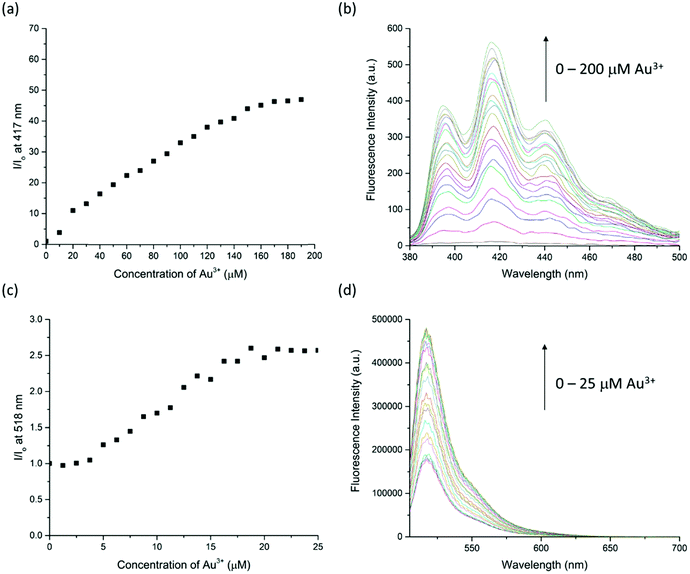
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v/v) had more than 50-fold enhancement on fluorescence intensity at 417 nm with 10.0 equiv. of Au3+. The fluorescence response has significantly improved and can only be turned on by Au3+ (Fig. 4). This suggests that Au3+ could inhibit the PET of RRA. In comparison with RRA, the response of RRB has 1.2-fold enhancement upon the addition of 10.0 equiv. of Au3+ and 0.6-fold enhancement upon the addition of 10.0 equiv. of Hg2+ and Cu2+ separately in MeCN/H2O solution (50
50, v/v/v) had more than 50-fold enhancement on fluorescence intensity at 417 nm with 10.0 equiv. of Au3+. The fluorescence response has significantly improved and can only be turned on by Au3+ (Fig. 4). This suggests that Au3+ could inhibit the PET of RRA. In comparison with RRA, the response of RRB has 1.2-fold enhancement upon the addition of 10.0 equiv. of Au3+ and 0.6-fold enhancement upon the addition of 10.0 equiv. of Hg2+ and Cu2+ separately in MeCN/H2O solution (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v). Next, the metal interference on Au3+ detection was tested. To study the interference, RRA and RRB were added to a mixture of Au3+ and one of the metal ions used in the previous experiment. The fluorescence response of rotaxanes to Au3+ is similar in the presence of most of the metal ions except for Pd2+, Ru3+ and V3+, suggesting that the interference from other metal ions was insignificant (Fig. 4b). The quenching of Pd2+, Ru3+ and V3+ may be due to the similar size with Au3+, having an effective ionic radius of 86, 68, 64 and 85 pm respectively.
50, v/v). Next, the metal interference on Au3+ detection was tested. To study the interference, RRA and RRB were added to a mixture of Au3+ and one of the metal ions used in the previous experiment. The fluorescence response of rotaxanes to Au3+ is similar in the presence of most of the metal ions except for Pd2+, Ru3+ and V3+, suggesting that the interference from other metal ions was insignificant (Fig. 4b). The quenching of Pd2+, Ru3+ and V3+ may be due to the similar size with Au3+, having an effective ionic radius of 86, 68, 64 and 85 pm respectively.
Since both RRA and RRB show high selectivity to Au3+, NMR titration of Au3+ was performed and illustrated (Fig. 5). Upon addition of Au3+, the 1H NMR spectra of RRA and RRB changed dramatically, exhibiting a similar pattern to the acid-equilibrium form of both RRA and RRB. Moreover, the appearance of ammonium protons (Hc) also proved that the dialkylamine of RRA (Hcδ 8.89 ppm) and RRB (Hcδ 8.99 ppm) was protonated by Au3+. During the titration, chemical shifts of the pyridyl (Ha,b), ammonium proton (Hc) and phenyl (Hd–g) units which belong to the macrocyclic ring were observed, indicating their participation in the Au3+ complexation. Since the proton signal of both the axle and macrocycle has shifted during the complexation, we proposed that the Au3+ was bound inside the cavity of the rotaxanes (Scheme 3).
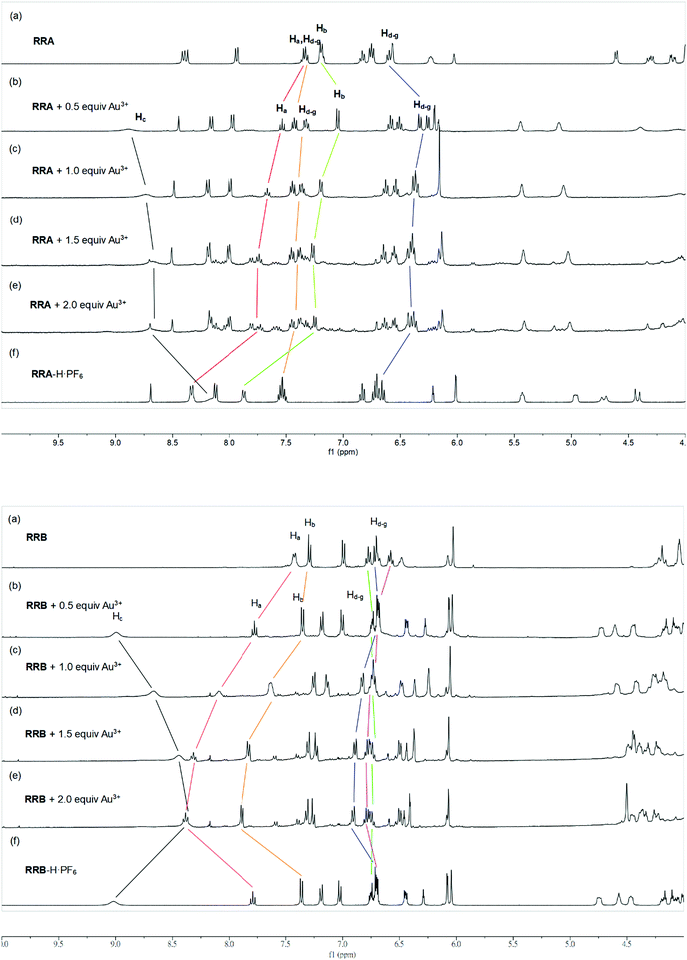 | ||
| Fig. 5 Stacked 1H NMR spectra (CD3CN, 400 MHz, 298 K) of RRA (upper) and RRB (lower) upon addition of (a) 0, (b) 0.5, (c) 1.0 (d) 1.5, and (e) 2.0 equiv. of Au3+. (f) RRA-H·PF6, and RRB-H·PF6. | ||
The sensitivity of Au3+ detection was studied by a fluorescence titration experiment (Fig. 6). Upon titration, the fluorescence intensity of RRA and RRB is linearly proportional to the increase of Au3+ concentration in the range of 0–160 μM and 5–20 μM, respectively. The intensity remains steady after the addition of 160 μM of Au3+ to RRA solution and 20 μM of Au3+ to RRB. The results indicate that the amount of Au3+ can be estimated by both rotaxane sensors. Indeed, the binding constant (Ka) with RRA to Au3+ is 9.05 × 103 M−1 and that of RRB with Au3+ is 2.92 × 104 M−1, calculated by using the equation Ka = B−1 × [Imax − Imin]−1 from Benesi–Hildebrand analysis (Fig. S2 and S3, ESI†). This reveals that RRB can bind stronger to Au3+ than RRA.
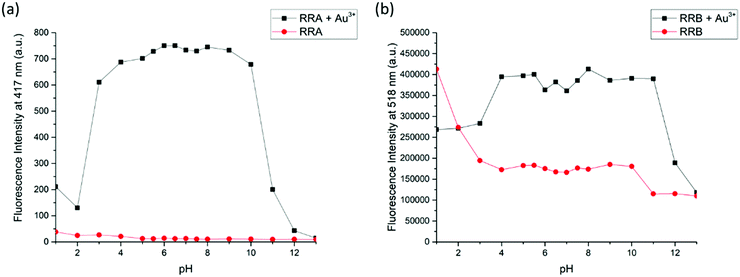
Furthermore, because the NMR titration indicates that RRA and RRB were acidified during the binding to Au3+, the fluorescence response of RRA and RRB to Au3+ can be changed according to the pH equilibrium between the rotaxanes and their protonated form. Therefore, the fluorescence responses of RRA and RRB were tested at different pH values (Fig. 7). Without Au3+, RRA has weak fluorescence in the pH range of 1.0–13.0. While the fluorescence of RRA was turned on significantly from pH 4.0–10.0 upon addition of Au3+. The results show that the Au3+ detection is partially pH independent with RRA when the pH is in the range of 4.0–10.0. For RRB, it gives weak fluorescence in the pH range of 3.0–13.0 without Au3+. Upon addition of Au3+, fluorescence was turned on significantly from pH 4.0–11.0. Under extreme acidic conditions, the pyridyl and amine units were protonated, which affects the binding to Au3+. Moreover, BODIPY was unstable and could not give reliable sensing results based on fluorescence signals at extremely acidic conditions (pH < 2).42 Under extreme alkali conditions, the environment facilitates the formation of gold hydroxide, which has a low water solubility (Ksp = 5 × 10−46). Without dissolving gold ions in the solution, the formation of a sensor-metal complex is slow, so the fluorescence response of the rotaxanes is greatly reduced.
In addition to the titration experiment, the response time experiment was also conducted to investigate the sensitivity of RRA and RRB to Au3+ (Fig. S4, ESI†). The results show that the fluorescence intensity became steady around 15 min and 5 min for RRA and RRB respectively, suggesting that both sensors can be used for quick recognition. Moreover, the limit of detection (LOD) of RRA for Au3+ is 3.23 μM and RRB for Au3+ is 0.35 μM according to equation: LOD = 3σ/m.
The cell viability of HeLa cells treated with RRA and RRB for 24 h was assessed using CCK-8 (Fig. S5, ESI†). As a result, the two sensors presented different toxic effects on HeLa cells. For RRA treated cells, the viability showed a remarkable decline at 0.2 μM, while the viability in RRB treated cells only displayed a slight suppression at 2.0 μM.
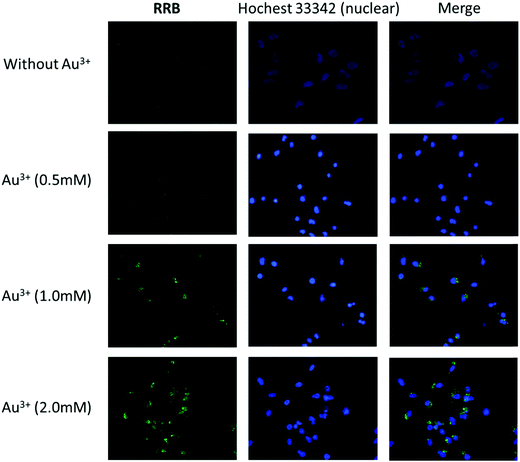
Since RRA was toxic to the cells even at low concentration (0.2 μM), we only examined the ion detection ability using RRB in HeLa cells. It was found that RRB could stain the cells and show weak green fluorescence without Au3+ treatment (Fig. 8). After the pre-treatment of Au3+, the green fluorescence in the cells was obviously enhanced along with the increase of Au3+ concentration. Interestingly, the green fluorescence was mostly found in the vacuole-like organelles appearing as green light spots under a fluorescent microscope. This result suggests that RRB could detect Au3+ and present an ion concentration-dependent sensing capacity in HeLa cells.
Conclusion
In conclusion, we have synthesized new fluorescent [2]rotaxane sensors. The fluorescence properties of rotaxanes in metal ion detection have been demonstrated. Dynamic rotaxane RA-H·PF6 can be hydrolyzed and its fluorescence switched on by common trivalent metal ions. For the reduced form of dynamic rotaxanes, RRA and RRB have been applied to detect Au3+ in 50% H2O in MeCN as a turn-on fluorescent sensor, and the metal interference on Au3+ detection is insignificant. Moreover, the fluorescence intensity of rotaxanes is linearly proportional to the concentration of Au3+ until excess. RRB can be used for Au3+ imaging in HeLa cells, demonstrating the application of using mechanically interlocked molecules as imaging probes in biological systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Professor Sir Fraser Stoddart (Department of Chemistry, Northwestern University) for helpful discussions. We thank Dr Xuan Li and Prof. Lijian Jin (Faculty of Dentistry, University of Hong Kong) for the biological studies. We thank Dr Tao Wang (HKBU) for the ESI-MS analysis. This work was partially supported by The State Key Laboratory of Environmental and Biological Analysis as well as The President's Award for Outstanding Performance in Research Supervision to K. C.-F. L. from The Hong Kong Baptist University.References
- R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936 RSC.
- X. Zhang, J. Yin and J. Yoon, Chem. Rev., 2014, 114, 4918 CrossRef CAS PubMed.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105 RSC.
- N. De Acha, C. Elosúa, M. J. Corres and J. F. Arregui, Sensors, 2019, 19 Search PubMed.
- J. F. Stoddart and H. M. Colquhoun, Tetrahedron, 2008, 64, 8231 CrossRef CAS.
- C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2016 Search PubMed.
- M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935 CrossRef CAS PubMed.
- M. Cirulli, A. Kaur, J. E. M. Lewis, Z. Zhang, J. A. Kitchen, S. M. Goldup and M. M. Roessler, J. Am. Chem. Soc., 2019, 141, 879 CrossRef CAS PubMed.
- K. Hiratani, M. Kaneyama, Y. Nagawa, E. Koyama and M. Kanesato, J. Am. Chem. Soc., 2004, 126, 13568 CrossRef CAS PubMed.
- M. Denis, J. Pancholi, K. Jobe, M. Watkinson and S. M. Goldup, Angew. Chem., Int. Ed., 2018, 57, 5310 CrossRef CAS PubMed.
- L. M. Hancock, L. C. Gilday, S. Carvalho, P. J. Costa, V. Félix, C. J. Serpell, N. L. Kilah and P. D. Beer, Chem. – Eur. J., 2010, 16, 13082 CrossRef CAS PubMed.
- T. Shukla, A. K. Dwivedi, R. Arumugaperumal, C. M. Lin, S. Y. Chen and H. C. Lin, Dyes Pigm., 2016, 131, 49 CrossRef CAS.
- X. Wang, J. Zhu and D. B. Smithrud, J. Org. Chem., 2010, 75, 3358 CrossRef CAS PubMed.
- S.-Y. Hsueh, C.-C. Lai and S.-H. Chiu, Chem. – Eur. J., 2010, 16, 2997 CrossRef CAS PubMed.
- W. Zhou, J. Li, X. He, C. Li, J. Lv, Y. Li, S. Wang, H. Liu and D. Zhu, Chem. – Eur. J., 2008, 14, 754 CrossRef CAS PubMed.
- R. Arumugaperumal, V. Srinivasadesikan, M. V. Ramakrishnam Raju, M.-C. Lin, T. Shukla, R. Singh and H.-C. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 26491 CrossRef CAS PubMed.
- J. Y. C. Lim, I. Marques, V. Félix and P. D. Beer, J. Am. Chem. Soc., 2017, 139, 12228 CrossRef CAS PubMed.
- J. Y. C. Lim, I. Marques, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2018, 57, 584 CrossRef CAS PubMed.
- Y.-C. Yeh, B. Creran and V. M. Rotello, Nanoscale, 2012, 4, 1871 RSC.
- C. F. Shaw, Chem. Rev., 1999, 99, 2589 CrossRef CAS.
- I. Ott, Coord. Chem. Rev., 2009, 253, 1670 CrossRef CAS.
- A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed.
- D. Pflästerer and A. S. K. Hashmi, Chem. Soc. Rev., 2016, 45, 1331 RSC.
- R. Ciriminna, E. Falletta, C. Della Pina, J. H. Teles and M. Pagliaro, Angew. Chem., Int. Ed., 2016, 55, 14210–14217 CrossRef CAS.
- A. Habib and M. Tabata, J. Inorg. Biochem., 2004, 98, 1696 CrossRef CAS PubMed.
- A. Kundu, R. K. Layek, A. Kuila and A. K. Nandi, ACS Appl. Mater. Interfaces, 2012, 4, 5576 CrossRef CAS PubMed.
- E. E. Connor, J. Mwamuka, A. Gole, C. J. Murphy and M. D. Wyatt, Small, 2005, 1, 325 CrossRef CAS PubMed.
- P. T. Glink, A. I. Oliva, J. F. Stoddart, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2001, 40, 1870 CrossRef CAS PubMed.
- M. Horn, J. Ihringer, P. T. Glink and J. F. Stoddart, Chem. – Eur. J., 2003, 9, 4046 CrossRef CAS PubMed.
- J. Wu, K. C.-F. Leung and J. F. Stoddart, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17266 CrossRef CAS.
- M. E. Belowich, C. Valente and J. F. Stoddart, Angew. Chem., Int. Ed., 2010, 49, 7208 CrossRef PubMed.
- A.-J. Avestro, D. M. Gardner, N. A. Vermeulen, E. A. Wilson, S. T. Schneebeli, A. C. Whalley, M. E. Belowich, R. Carmieli, M. R. Wasielewski and J. F. Stoddart, Angew. Chem., Int. Ed., 2014, 53, 4442 CrossRef CAS PubMed.
- Z. Li, X. Han, H. Chen, D. Wu, F. Hu, S. H. Liu and J. Yin, Org. Biomol. Chem., 2015, 13, 7313 RSC.
- X. Han, Z. Li, Z. Xu, Z. Zhao, S. H. Liu and J. Yin, Chin. J. Chem., 2017, 35, 1050 CrossRef CAS.
- Z. Li, C. He, Y. Li, Y. Wang, C. Wei, J. Yin and S. H. Liu, Dyes Pigm., 2018, 148, 130 CrossRef CAS.
- Y.-H. Chang, Y.-J. Lee, C.-C. Lai, Y.-H. Liu, S.-M. Peng and S.-H. Chiu, Org. Lett., 2018, 20, 2416 CrossRef CAS PubMed.
- V. Dvornikovs, B. E. House, M. Kaetzel, J. R. Dedman and D. B. Smithrud, J. Am. Chem. Soc., 2003, 125, 8290 CrossRef CAS PubMed.
- A. Fernandes, A. Viterisi, F. Coutrot, S. Potok, D. A. Leigh, V. Aucagne and S. Papot, Angew. Chem., Int. Ed., 2009, 48, 6443 CrossRef CAS PubMed.
- R. Barat, T. Legigan, I. Tranoy-Opalinski, B. Renoux, E. Péraudeau, J. Clarhaut, P. Poinot, A. E. Fernandes, V. Aucagne, D. A. Leigh and S. Papot, Chem. Sci., 2015, 6, 2608 RSC.
- G. Yu, D. Wu, Y. Li, Z. Zhang, L. Shao, J. Zhou, Q. Hu, G. Tang and F. Huang, Chem. Sci., 2016, 7, 3017 RSC.
- W.-Y. Wong, K. C.-F. Leung and J. F. Stoddart, Org. Biomol. Chem., 2010, 8, 2332 RSC.
- M. Baruah, W. Qin, C. Flors, J. Hofkens, R. A. L. Vallée, D. Beljonne, M. Van der Auweraer, W. M. De Borggraeve and N. Boens, J. Phys. Chem. A, 2006, 110, 5998 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1942423. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qm00476a |
| This journal is © the Partner Organisations 2019 |