A novel Cu-nanowire@Quasi-MOF via mild pyrolysis of a bimetal-MOF for the selective oxidation of benzyl alcohol in air†
Abstract
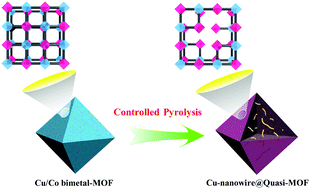
The preparation of metal nanocomposite catalysts with MOFs as precursors has attracted extensive attention, as the design and preparation of catalysts by this method could maximize the advantages of the precursors and ensure the stability of the catalysts as much as possible. Here, a benzimidazole-modified Cu/Co bimetal-MOF as a precursor was used to synthesize a Cu-nanowire@Quasi-MOF material via a simple and low-cost thermal decomposition strategy for the first time. Experimental evaluation suggested that the Co-MOF part in Cu/Co bimetal-MOF was a morphology retainer, and the special channel composition of the Quasi-MOF played a space-confined role in the formation of the unique Cu-nanowires. Based on the detailed investigation, a possible formation mechanism was proposed. Furthermore, Cu-nanowire@Quasi-MOF exhibited excellent catalytic performance and good stability in the selective oxidation of benzyl alcohol in air. This study demonstrated that using MOFs as a precursor, by adjusting the calcination temperature and adding heteroatoms, can control the morphology of metal nanoparticles (MNPs) in MOF derivatives, reduce agglomeration and enhance catalytic activity, which will be a promising approach to in situ preparation of complex metal nanocomposites.
- This article is part of the themed collection: Celebrating the 100th anniversary of Nankai University


 Please wait while we load your content...
Please wait while we load your content...