AliBu3: unprecedented main-group metal catalyst for helical sense-selective polymerization of chiral aryl isocyanides and copolymerization with achiral aryl isocyanides†
Abstract
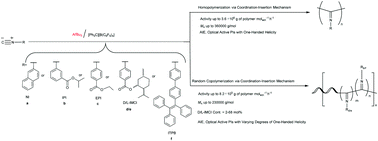
The polymerization of isocyanide in screw-sense usually proceeds via a late-transition metal-catalyzed coordination-insertion mechanism or a metal-free cation-initiated carbocationic mechanism. By contrast, less attention has been paid to main-group metal catalysts in isocyanide polymerization, and no main-group metal catalyst has exhibited high activity and helical sense selectivity in isocyanide polymerization till date. In the presence of [Ph3C][B(C6F5)4], AliBu3, which is a commercially available, cheap, simple and common main-group organometallic reagent, represents the first example of a highly efficient single-site main-group metal catalyst for the polymerization of several aryl isocyanides bearing polar, chiral and tetraphenylethylene (TPE) substituents, showing very high activities up to 3.6 × 106 g polymer molact.−1 h−1. Moreover, this catalytic system also catalyzes the homopolymerization of chiral isocyanides and the copolymerization between chiral isocyanides and achiral isocyanides in screw-sense to form high molecular weight polyisocyanides with varying degrees of single-handed helical conformation and AIE nature. Based on in situ1H NMR, IR, and ESI-MS spectroscopy, a cationic Al alkyl active species and a possible coordination-insertion (co)polymerization mechanism are proposed.
- This article is part of the themed collection: 2019 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...