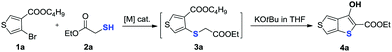Divergent synthesis of 3-substituted thieno[3,4-b]thiophene derivatives via hydroxy-based transformations†
Yue
Zhou
,
Jie
Hao
and
Dongbing
Zhao
 *
*
State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, 94 Weijin Road, Tianjin 300071, China. E-mail: dongbing.chem@nankai.edu.cn
First published on 6th May 2019
Abstract
Herein, we have developed the first Pd-catalytic method for the preparation of 3-hydroxythieno[3,4-b]thiophene-2-carboxylate, which can be widely utilized as the chemical building block for modular assembly of structurally diverse 3-substituted thieno[3,4-b]thiophene derivatives via hydroxy-based transformation. Furthermore, we have evaluated the effect on their photophysical properties of the introduction of different substitutions at the C3-position and prepared conjugated polymers bearing 3-substituted thieno[3,4-b]thiophene units. We demonstrated that the photoluminescence (PL) properties of thieno[3,4-b]thiophene-2-carboxylate can be efficiently modulated by introducing different functional groups at the C3-position.
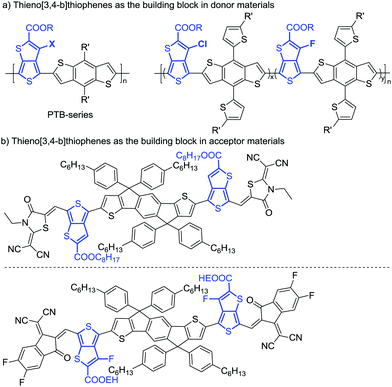
As is well recognized, thiophene-based conjugated molecules have played an indispensable role in the development of organic optoelectronics.1 Among various thiophene building blocks, the thieno-[3,4-b]thiophene (TT) unit with functional groups has recently emerged as an extremely attractive electron-withdrawing building block in organic electronics.2 In particular, thieno[3,4-b]thiophenes (TbTs) bearing a carboxyl group have been widely used in the development of donor and acceptor materials in organic solar cells (OSCs) represented by the PTB series family polymers and IDT-derived non-fullerene small molecules acceptors with state-of-the-art power conversion efficiencies (PCEs) (Scheme 1).3–5 Thieno[3,4-b]thiophene (TbT) is an asymmetric fused bithiophene containing four functionalization positions, in which the proaromatic thiophene can effectively stabilize the quinoidal resonance of the aromatic thiophene, narrow the energy gap, and modulate the electronic structures of the resulting molecules.
 | ||
| Scheme 1 Representative donor and acceptor materials bearing thieno[3,4-b]thiophene-2-carboxylate units in organic solar cells. | ||
Historically, synthesis of thieno[3,4-b]thiophenes (TbTs) bearing a carboxyl group involved: (1) Cu-mediated coupling between 4-bromothiophene-3-carbaldehyde and 2-mercaptoacetate (Scheme 2a).6 However, this method is suitable only for the preparation of 3-unsubsituted thieno[3,4-b]thiophenes; (2) the non-catalytic multi-step cyclization reaction from 3-substituted thiophene-2-carboxylate (Scheme 2b).7 Even though this method has the capacity to access 3-substituted thieno[3,4-b]thiophenes, it still suffers from disadvantages such as linear multi-step synthesis (more than 5 steps needs for each product), low yields, harsh reaction conditions, and limited substrate scopes. Thus, development of a methodology to access thieno[3,4-b]thiophenes with various substitution patterns at the 3-position in a rapid and modular manner from readily available precursors is highly appealing. Herein, we have developed the first Pd-catalytic method for the preparation of 3-hydroxythieno[3,4-b]thiophene-2-carboxylate from commercially available 4-bromothiophene-3-carboxylate and 2-mercaptoacetate (Scheme 2c), which has never been prepared before our study. We demonstrated that this 3-hydroxythieno[3,4-b]thiophene-2-carboxylate can be widely utilized as a chemical building block for modular assembly of structurally diverse 3-substituted thieno[3,4-b]thiophene derivatives via a wide range of hydroxy-based transformations. Moreover, we have evaluated the effect on their photophysical properties of the introduction of different substitutions at the C3-position and then developed a series of TT-based conjugated polymer donors.
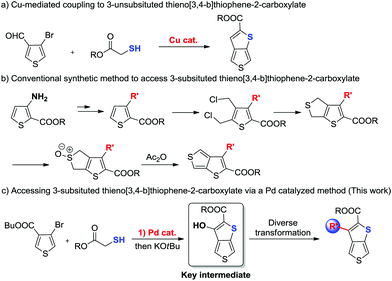 | ||
| Scheme 2 The conventional synthetic methods to access thieno[3,4-b]thiophene-2-carboxylates and our study. | ||
In our preliminary study, the reaction of butyl 4-bromothiophene-3-carboxylate 1a with ethyl 2-mercaptoacetate 2a was examined under Cu-catalytic conditions. Unfortunately, the developed Cu-catalytic system for the preparation of 3-unsubstituted thiophene-2-carboxylate was not effective for this conversion (Table 1, entries 1–3). After an extensive screening such as different Pd0 sources, solvents, reaction times and ligands, we found that treatment of 1a (0.2 mmol) and 2a with Pd2dba3 (5 mol%), xantphos (10 mol%) in toluene (1 mL) at 120 °C for 24 h gave product 3a in 97% yield (Table 1, entry 4). Evaluation of different bases revealed that DIPEA was the best choice (entries 3–7). There is no reactivity at all in the absence of Pd catalyst (entry 8). Finally, we demonstrated that the product 3a can be smoothly cyclized to form the key intermediate 3-hydroxythieno[3,4-b]thiophene-2-carboxylate (entry 4) with 81% yield in the presence of KOtBu in THF.
| Entry | Base | Catalyst | Ligand | Solvent | Temp. (°C) | Yieldb |
|---|---|---|---|---|---|---|
| a Reactions were carried out with [M] catalyst (10 mol%), ligand (10 mol%), base (1.5 equiv.), 1a (0.2 mmol), and ethyl 2-mercaptoacetate (2a, 0.4 mmol) in solvent (1 mL) for 24 h under a N2 atmosphere. b Yields were determined by isolation. N.R.: no reaction. | ||||||
| 1 | K2CO3 | CuO | — | DMSO | 60 | N.R. |
| 2 | K2CO3 | CuO | — | DMSO | 140 | N.R. |
| 3 | K2CO3 | CuO | Phen | DMSO | 140 | N.R. |
| 4 | DIPEA | Pd 2 dba 3 | Xantphos | Toluene | 120 | 81 |
| 5 | K2CO3 | Pd2dba3 | Xantphos | Toluene | 120 | 61 |
| 6 | KOtBu | Pd2dba3 | Xantphos | Toluene | 120 | 21 |
| 7 | NaOtBu | Pd2dba3 | Xantphos | Toluene | 120 | 24 |
| 8 | DIPEA | — | Xantphos | Toluene | 120 | N.R. |
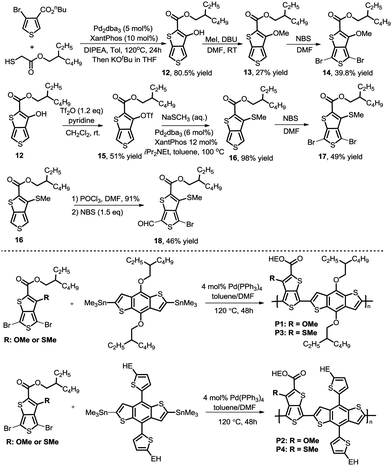
As we proposed, the product 3-hydroxythieno[3,4-b]thiophene-2-carboxylate 4a obtained by the method described herein could provide access to structurally diverse 3-substituted thiophene-2-carboxylates by means of additional hydroxy-based transformations (Scheme 3).8 First, the hydroxy group of 4a was easily methylated to yield the 3-methoxythieno[3,4-b]thiophene-2-carboxylate 5 using methyl iodide as the methylation reagent. 3-Methoxythieno[3,4-b]thiophene-2-carboxylate 5 could further undergo bromination in the presence of NBS in DMF to yield compound 6 with 60% yield. Furthermore, the hydroxy group of 4a could also been smoothly transformed to –OTf in the presence of Tf2O/pyridine, which is a versatile leaving group in a lot of different types of cross-coupling reactions.9 For example, the –OTf of 7 can couple with NaSCH3 under Pd-catalytic conditions to produce compound 8 in 91% yield. In addition, the amination of product 7 with diphenylmethanimine in the presence of a Pd0 catalyst and K3PO4 in toluene also worked smoothly to afford the corresponding 9 in 86% yield. Product 7 could also undergo Suzuki coupling to yield 3-phenylthieno[3,4-b]thiophene-2-carboxylate 10 in 97% yield. Pd-Catalyzed Sonogashira coupling of product 7 and ethynylbenzene also smoothly occurred to access 3-alkynylthieno[3,4-b]thiophene-2-carboxylate 11 with 91% yield.
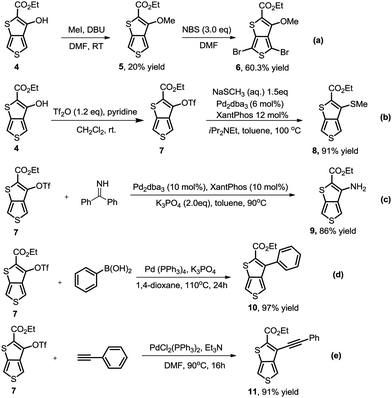 | ||
| Scheme 3 Accessing structurally diverse 3-substituted thiophene-2-carboxylates by means of additional hydroxy-based transformations. | ||
After development of the modular methodology to access diverse 3-substituted thieno[3,4-b]thiophene-2-carboxylates, we aimed to introduce these building blocks into conjugated polymers. Following the procedures developed, we synthesized the 3-methoxy and 3-methylthio TbT monomers with a branched alkyl chain to guarantee enough solubility of the polymers. The synthetic route is described in Scheme 4 (upper panel). Furthermore, we found that 4-bromo-6-formyl-3-substituted [3,4-b]thiophene-2-carboxylate 18 could been produced by regioselective monoformylation and sequential bromination, which can be further utilized in the development of new small molecule donors and acceptors.10 Then, a series of conjugated polymers were prepared by Stille coupling reactions of the TbT monomers and BDT-T or BDT,11 namely P1, P2, P3, and P4 (Scheme 4, lower panel). Detailed synthetic routes and characterizations can be found in the Experimental section of the ESI.†
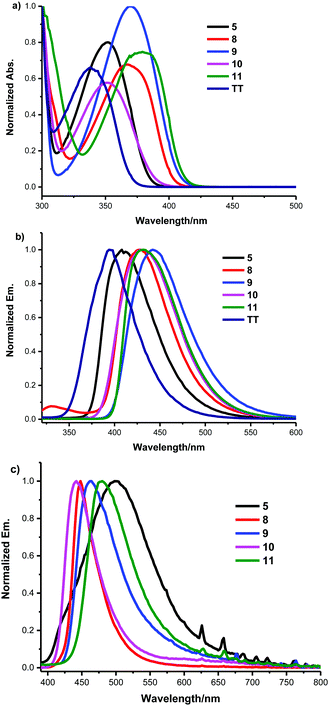
We first evaluated the effect of the subsitution at the C3-position of thieno[3,4-b]thiophene-2-carboxylate on their photophysical properties. The absorption maxima, photoluminescence (PL) emission maxima, and PL quantum yields (Φf) of compounds TT, 5, 8, 9, 10 and 11 in both the solid state and CH2Cl2 solution are listed in Table 2, whereas the corresponding absorption, and emission spectra are depicted in Fig. 1 and Fig. S1 (ESI†). It is worth noting that all these compounds were stable to light, humidity and air. As shown in Fig. 1, the subsitution at the C3-position was observed to greatly influence the molecular absorption and fluorescence. By incorporation of functional groups into the C3-position, all of the absorption and emission peaks of these derivatives were obviously red-shifted and their PL efficiencies were also enhanced in comparison with the ethyl[3,4-b]thiophene-2-carboxylate (TT) in both the solid state and CH2Cl2 solution. This might stem from the restriction of intramolecular rotation (RIR) of the ester group at the C2-position and substitution at the C3-position. Fig. 1b and c reveal that the emission peaks of these monomers in thin films are different from their emissions in CH2Cl2. This likely indicates that these monomers involved different intermolecular interactions and molecular aggregation in the solid state and in the dilute solution state.
| Comp. | UV-Vis absorption | Emission | |||
|---|---|---|---|---|---|
| λ solmax | λ filmmax | λ solmax | λ solidmax | Φ sol (%) | |
| a Absolute quantum yield determined with an integrating sphere system. n.d. = not detected due to the weak emission intensity. | |||||
| TT | 338 | 352 | 357 | n.d. | n.d. |
| 5 | 352 | 388 | 409 | 499 | 3.42 |
| 8 | 368 | 400 | 426 | 447 | 0.71 |
| 9 | 369 | 400 | 442 | 462 | 88 |
| 10 | 352 | 359 | 429 | 440 | 9.64 |
| 11 | 378 | 397 | 430 | 478 | 6.5 |
The optical properties of these conjugated polymers bearing 3-methoxy and 3-methylthio TbT units in solution and in thin films were then also characterized by UV-Vis absorption spectroscopy as shown in Fig. 2. The corresponding spectroscopic data are summarized in Table 3. In general, the absorption maxima of these polymers bearing 3-methoxy or 3-methylthio[3,4-b]thiophene-2-carboxylate in solution and in films showed blue-shifts compared with the related polymers, which did not have substitution at the C3-position.12 This means that the steric hindrance of these substitutions at the C3-position likely disrupted the conjugation of the polymer chain. Thiomethylation of the polymers lead to larger blue-shifts in the absorption spectra compared to methoxylation of the polymers.2,13 On the other hand, Fig. 2a and b reveal that the absorption peaks of the polymers in thin films are slightly red-shifted compared to their absorptions in CHCl3. This likely indicates that the polymers form slightly stronger intermolecular interactions and molecular aggregation in the solid film than in the dilute solution state. Finally, gel permeation chromatography (GPC) was also measured to obtain the molecular weights. It showed average Mn of 14.4, 10.8, 21.2, and 12.3 kDa for P1, P2, P3, and P4, respectively. The polydispersity index (PDI) of these polymers was determined to be about 1.8–2.1. Notably, all the polymers are soluble in chloroform (CHCl3), chlorobenzene (CB), and 1,2-dichlorobenzene (DCB).
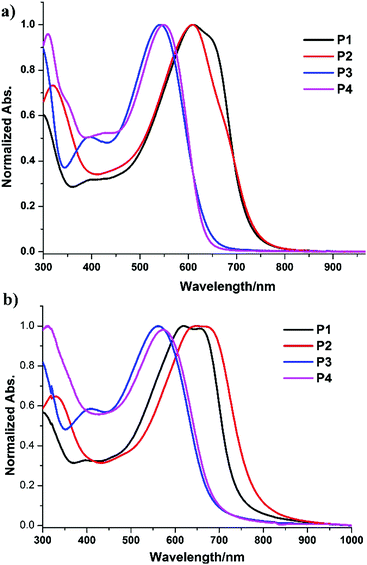 | ||
| Fig. 2 Normalized UV/Vis absorption spectra of P1, P2, P3, and P4 in a chloroform solution (a) and as a solid film (b). | ||
| P | UV-Vis absorption | CVc | GPCd | ||||
|---|---|---|---|---|---|---|---|
| λ max | λ max |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
LUMO | HOMO | M n (kD) | PDI | |
| Sol | Film | Film | |||||
| a Determined from UV-Vis absorption spectroscopy in CHCl3 and as a thin film. b Estimated from the onset wavelength of the optical absorption: Eoptg = 1240/λedge. c Vs. Ag/Ag+ in acetonitrile solution with 0.1 M n-Bu4NPF6 as a supporting electrolyte. d Molecular weights measured using gel permeation chromatography (against polystyrene standards) in chlorobenzene at 80 °C. | |||||||
| P1 | 608 | 637 | 1.65 | 3.43 | 5.08 | 14.4 | 2.1 |
| P2 | 619 | 660 | 1.56 | 3.47 | 5.03 | 10.8 | 1.9 |
| P3 | 541 | 561 | 1.82 | 3.46 | 5.28 | 21.2 | 1.9 |
| P4 | 549 | 572 | 1.80 | 3.46 | 5.26 | 12.3 | 2.1 |
From the absorption onset in films, the optical bandgaps are evaluated to be 1.65, 1.56, 1.82, and 1.80 eV for P1, P2, P3, and P4, respectively. In general, the introduction of a thiomethyl group led to significant broader optical bandgaps. To further study the energy level and band gap of these polymers, electrochemical cyclic voltammetry (CV) has also been employed to measure their redox properties (Fig. S2 and S3 in the ESI†). On incorporation into the reductive onset potentials and the optical bandgaps, the LUMO/HOMO energy levels are estimated to be −3.43/−5.08 eV (P1), −3.47/−5.03 eV (P2), −3.46/−5.28 eV (P3), and −3.46/−5.26 eV (P4).
Conclusions
In conclusion, we have developed the first Pd-catalytic method for the preparation of 3-hydroxythieno[3,4-b]thiophene-2-carboxylate, which has never been prepared before our study. Furthermore, we demonstrated that 3-hydroxythieno[3,4-b]thiophene-2-carboxylate can be employed as a versatile chemical building block for modular assembly of structurally diverse 3-substituted thieno[3,4-b]thiophene derivatives via hydroxy-based transformations. Additionally, the optical and electrochemical properties of these 3-substituted thieno[3,4-b]thiophene derivatives were also investigated. The results revealed that the photoluminescence (PL) properties of thieno[3,4-b]thiophene-2-carboxylate can be efficiently modulated by introducing different functional groups at the C3-position. Finally, several conjugated polymers bearing 3-methoxy and 3-methylthio TbT units were also synthesized and characterized. The results indicated that we can efficiently tune the energy levels, the optical bandgaps and absorption spectra by introducing 3-substituted thieno[3,4-b]thiophene derivatives into polymers. The device fabrication, measurements and optimization are currently underway in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (21871146 and 21602115), 1000-Talent Youth Program (020/BF180181), the Natural Science Foundation of Tianjin (18JCYBJC20400), the Fundamental Research Funds for the Central Universities and Nankai University.Notes and references
- (a) I. F. Perepichka and D. F. Perepichka, Handbook of Thiophene-based Materials: Applications in Organic Eletronics and Photonics, Wiley-VCH, Weinheim, 2009 CrossRef; (b) H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397 CrossRef CAS PubMed; (c) A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed; (d) M. E. Cinar and T. Ozturk, Chem. Rev., 2015, 115, 3036 CrossRef CAS.
- C. Zhang and X. Zhu, Acc. Chem. Res., 2017, 50, 1342 CrossRef CAS PubMed.
- For some examples on the PTB series family polymers as the donors in OSCs, see: (a) Y. Liang, Y. Wu, D. Feng, S.-T. Tsai, H.-J. Son, G. Li and L. Yu, J. Am. Chem. Soc., 2009, 131, 56 CrossRef CAS PubMed; (b) Y. Liang, D. Feng, Y. Wu, S.-T. Tsai, G. Li, C. Ray and L. Yu, J. Am. Chem. Soc., 2009, 131, 7792 CrossRef CAS PubMed; (c) J. Hou, H.-Y. Chen, S. Zhang, R. I. Chen, Y. Yang, Y. Wu and G. Li, J. Am. Chem. Soc., 2009, 131, 15586 CrossRef CAS PubMed; (d) Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, 135 CrossRef PubMed; (e) Y. Huang, X. Guo, F. Liu, L. Huo, Y. Chen, T. P. Russell, C. C. Han, Y. Li and J. Hou, Adv. Mater., 2012, 24, 3383 CrossRef CAS PubMed; (f) Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS.
- For some examples on the organic small molecules bearing TbT units as the donors in OSCs, see: (a) F. Liu, G. L. Espejo, S. Qiu, M. M. Oliva, J. Pina, J. S. S. de Melo, J. Casado and X. Zhu, J. Am. Chem. Soc., 2015, 137, 10357 CrossRef CAS PubMed; (b) S. Xu, Z. Zhou, H. Fan, L. Ren, F. Liu, X. Zhu and T. P. Russell, J. Mater. Chem. A, 2016, 4, 17354 RSC.
- For some examples on the use of the TbT units in the development of non-fullerene acceptors in OSCs, see: (a) F. Liu, Z. Zhou, C. Zhang, T. Vergote, H.j. Fan, F. Liu and X. Zhu, J. Am. Chem. Soc., 2016, 138, 15523 CrossRef CAS PubMed; (b) F. Liu, Z. Zhou, C. Zhang, J. Zhang, Q. Hu, T. Vergote, F. Liu, T. P. Russell and X. Zhu, Adv. Mater., 2017, 29, 1606574 CrossRef PubMed; (c) F.-X. Chen, J.-Q. Xu, Z.-X. Liu, M. Chen, R. Xia, Y. Yang, T.-K. Lau, Y. Zhang, X. Lu, H.-L. Yip, A. K.-Y. Jen, H. Chen and C.-Z. Li, Adv. Mater., 2018, 30, 1803769 CrossRef PubMed.
- J. H. Park, Y. G. Seo, D. H. Yoon, Y.-S. Lee, S.-H. Lee, M. Pyo and K. Zong, Eur. Polym. J., 2010, 46, 1790 CrossRef CAS.
- (a) H. Wynberg and D. J. Zwanenburg, Tetrahedron Lett., 1967, 761 CrossRef CAS; (b) S. Y. Hong and D. S. Marynick, Macromolecules, 1992, 25, 4652 CrossRef CAS; (c) L. Huo, Z. Li, X. Guo, Y. Wu, M. Zhang, L. Ye, S. Zhang and J. Hou, Polym. Chem., 2013, 4, 3047 RSC; (d) S. Qu, H. Wang, D. Mo, P. Chao, Z. Yang, L. Li, L. Tian, W. Chen and F. He, Macromolecules, 2017, 50, 4962 CrossRef CAS.
- For selected reviews on hydroxy-based transformations, see: (a) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486 CrossRef CAS PubMed; (b) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS PubMed; (c) B.-J. Li, D.-G. Yu, C.-L. Sun and Z.-J. Shi, Chem. – Eur. J., 2011, 17, 1728 CrossRef CAS PubMed; (d) J. Yamaguchi, K. Muto and K. Itami, Eur. J. Org. Chem., 2013, 19 CrossRef CAS; (e) M. Tehetena and N. K. Garg, Org. Process Res. Dev., 2013, 17, 129 Search PubMed; (f) A. Correa, J. Cornella and R. Martin, Angew. Chem., Int. Ed., 2013, 52, 1878 CrossRef CAS PubMed; (g) M. Tobisu and N. Chatani, Top. Organomet. Chem., 2013, 44, 35 CrossRef CAS; (h) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC; (i) H. Zeng, Z. Qiu, A. Domínguez-Huerta, Z. Hearne, Z. Chen and C.-J. Li, ACS Catal., 2017, 7, 510 CrossRef CAS.
- Reviews on cross-coupling: (a) N. Miyaura, S. L. Buchwald, K. Fugami and T. Hiyama, Cross-Coupling Reactions: A Practical Guide, ed. N. Miyaura, Springer: Berlin, Germany, 2002 CrossRef; (b) F. Diederich and A. de Meijere, Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and A. de Meijere, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed; (c) A. Suzuki and Y. Yamamoto, Chem. Lett., 2011, 40, 894 CrossRef CAS.
- (a) Y.-C. Chen, H.-H. Chou, M. C. Tsai, S.-Y. Chen, J. T. Lin, C.-F. Yao and K. Chen, Chem. – Eur. J., 2012, 18, 5430 CrossRef CAS PubMed; (b) J. Duan, Y. Duan, Y. Zhao, B. He and Q. Tang, Chem. Commun., 2017, 53, 10046 RSC.
- (a) Y. Liang and L. Yu, Acc. Chem. Res., 2010, 43, 1227 CrossRef CAS PubMed; (b) H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397 CrossRef CAS PubMed.
- L. Huo, S. Zhang, X. Guo, F. Xu, Y. Li and J. Hou, Angew. Chem., Int. Ed., 2011, 50, 9697 CrossRef CAS PubMed.
- (a) D. Lee, E. Hubijar, G. Jones, D. Kalaw and J. P. Ferraris, Chem. Mater., 2012, 24, 2534 CrossRef CAS; (b) J. A. Schneider, A. Dadvand, W. Wen and D. F. Perepichka, Macromolecules, 2013, 46, 9231 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qm00128j |
| This journal is © the Partner Organisations 2019 |