Carbon-based materials for lithium-ion capacitors
Xiaojun
Wang
a,
Lili
Liu
b and
Zhiqiang
Niu
 *a
*a
aKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: zqniu@nankai.edu.cn
bTianjin Key Laboratory for Photoelectric Materials and Devices, School of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, China
First published on 27th March 2019
Abstract
Lithium-ion capacitors (LICs) can deliver high energy density, large power density and excellent stability since they possess a high-capacity battery-type electrode and a high rate capacitor-type electrode. Recently, great efforts have been devoted to fabricating carbon-based electrodes for LICs, which can effectively enhance their electrochemical performance due to the high electrochemical activity of carbon materials. Here, we present the recent developments of carbon-based materials as electrodes of LICs. Carbon-based battery-type materials, which can be either anodes or cathodes, highlighted here include carbonaceous materials, carbon/transition metal compounds, carbon/polyanion composites, carbon/metalloid and metal materials as anodes and carbon/metal compound composites as cathodes. Apart from battery-type electrodes, carbon-based materials also play an important role in the design of capacitor-type electrodes of LICs, which focus on carbonaceous materials as cathodes. The prospects and challenges in this field are also discussed.
1. Introduction
Lithium-ion batteries (LIBs) and supercapacitors (SCs) are considered as the two most promising energy storage systems.1–4 Typically, LIBs possess high energy density (>150 W h kg−1) but low power density (<1 kW kg−1) and inferior cycling stability (usually <4000 cycles).5–7 In contrast, SCs can provide large power density (>10 kW kg−1) as well as long cycle life (usually >105 cycles). However, the relatively poor energy density (5–10 W h kg−1) limits their applications in some fields.8,9 To bridge the gap between LIBs and SCs, lithium-ion capacitors (LICs) that can combine the merits of both LIBs and SCs were developed.10–14 A LIC device generally employs a high capacity battery-type electrode and a high rate capacitor-type electrode in combination with an appropriate electrolyte.15,16 During the charge–discharge process, charges are simultaneously and asymmetrically stored in the LIC by surface ion adsorption/desorption on the capacitor-type electrode and Li+ intercalation/de-intercalation in the battery-type electrode, respectively.17,18 In LICs, the capacitor-type electrode can be either the anode or cathode, while the battery-type electrode serves as the counter electrode. It is worth mentioning that the battery-type electrode and capacitor-type electrode perform in different potential windows, which can enlarge the operating voltage range of LICs and lead to high energy density.The energy and power densities of LICs mainly depend on the design of electrode materials in the devices.17,19,20 Until now, a variety of materials, such as metal compounds, polyanions and metalloid/metal compounds, have been fabricated for battery-type electrodes in LICs due to their high gravimetric specific capacity and excellent electrochemical activity.21 However, the low conductivity, large volume variation and high polarization of these active materials limit their further development in LICs.16 To solve these problems, carbon materials were often incorporated into the electrodes based on them due to their large specific surface area, high conductivity and outstanding electrolyte accessibility.22,23 In addition, carbon materials can also serve as the active materials of battery-type electrodes in LICs directly because they possess active sites for Li+ intercalation/de-intercalation.24–26 Different from battery-type electrodes, the counter electrodes of LICs are capacitor-type. As a result, various porous carbon materials with large specific surface area, such as activated carbon (AC), graphene and biomass-derived carbon, are promising candidates for capacitor-type electrodes of LICs.27,28 Their capacitances mainly depend on the ion adsorption/desorption on the surface of carbon-based electrodes.29 Thus, porous structures with an appropriate pore-size distribution play an important role in the electrochemical performance of carbon materials in LICs.30,31 To further enhance the capacitance of the capacitor-type electrode, functional groups are usually introduced into the carbonaceous materials to provide pseudo-capacitance.32,33 As discussed above, progress towards advanced LICs mostly benefits from the continuous development of carbon materials.
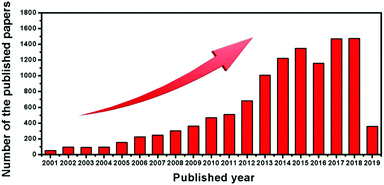
LICs were first developed by Amatucci et al. in 2001.34 Since then, the studies on LICs have flourished, as shown in Fig. 1. Although some reviews of energy-storage systems have mentioned the progress in LIC systems,3,23,25,35–38 a systematic review focusing on carbon-based materials for LICs has not been conducted. In this review, we will describe the fundamental principle of LICs and discuss the carbon-based battery-type electrode/capacitor-type electrode materials and their renaissance over several decades. Then we highlight the major roles of carbon, either as active materials or as conductive materials, in the applications of LICs by focusing on the most representative examples (Fig. 2). After a brief summary, the prospects and challenges associated with LICs are also discussed, which provide a deep insight into the carbon-based electrodes for future research of LICs.
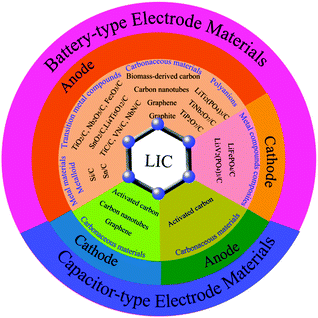 | ||
| Fig. 2 Schematic illustration of carbon-based battery-type electrodes and capacitor-type electrodes of LICs. | ||
2. The bases of lithium-ion capacitors
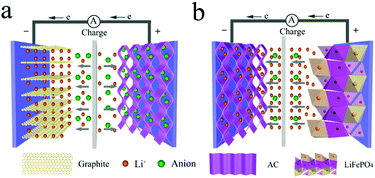
It is noteworthy that the lithium-ion capacitor (LIC) and the lithium-ion battery-type capacitor are collectively called a lithium-ion hybrid capacitor. LICs are electrochemical energy storage devices that combine the advantages of high power density of a supercapacitor and high energy density of a Li-ion battery. Before understanding the basic energy storage mechanism of LICs, it is necessary to identify the components of LICs. As a kind of asymmetric supercapacitor, LICs are usually composed of a battery-type electrode with the insertion/extraction of lithium ions and a capacitor-type electrode with pseudo-capacitance or adsorption/desorption of ions.37,39,40 Since the battery-type electrode can not only act as an anode, but also serve as a cathode, the LICs can be divided into two types:(1) the battery-type electrode acts as the anode and the capacitor-type electrode serves as the cathode, such as a Li4Ti5O12//AC system. Typically, during the charge process, anions are absorbed on the porous surface or defects of the cathode, while Li+ ions are intercalated into the active material of the anode. During discharging, the adsorbed anions are released from the cathode and the Li ions are de-intercalated from the anode, as illustrated in Fig. 3a.41,42
(2) the capacitor-type electrode acts as the anode and the battery-type electrode serves as the cathode, such as an AC//LiFePO4 system. Typically, during the charge process, Li+ de-intercalates from the cathode material and enters the electrolyte. At the same time, Li+ in the electrolyte migrates and adsorbs on the anode. The discharge process will be the reverse case, as shown in Fig. 3b.
Significantly, a battery-type electrode often undergoes a faradaic reaction with a relatively constant potential, which will provide a large specific capacitance (Fig. 4).43 When the battery-type electrode works as an anode, it usually has to be pre-lithiated before assembling a LIC, which could maintain the potential of the anode much closer to that of Li (∼0.1 V vs. Li/Li+).44,45 As a result, the working voltage of LICs can increase and even reach up to 4.5 V. The energy density of LICs can be calculated by the following equation:
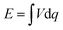 | (1) |
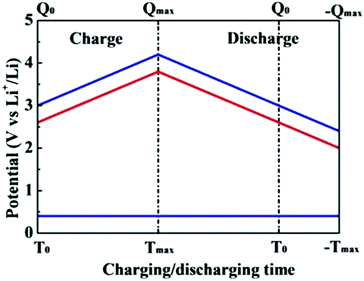 | ||
| Fig. 4 Schematic of voltage changes of asymmetrical cells with double-layer and intercalation electrodes and a capacitor-type anode during the charge–discharge process. The electrolytes with salts of LiPF6 are assumed to be used for asymmetrical cells, respectively. Reproduced with permission.43 Copyright 2009, Elsevier. | ||
Different from the battery-type electrode, a reversible ion adsorption or fast redox reaction occurs at the surface of the capacitor-type electrode, which offers a possibility that LICs possess comparable power density to that of SCs by combining a suitable battery-type electrode with fast kinetics.54,55 The cycling performance of LICs is also important for their practical application. In addition to the intrinsic behaviour of the battery-type electrode and capacitor-type electrode, the mass ratio between the battery-type electrode and capacitor-type electrode also plays a vital role in the cycle life of LICs. For an asymmetric cell, the charges at both electrodes should be balanced (Qanode = Qcathode). The stored charges are related to the specific capacity (C) and mass (m) of the electrode (Q = C × m).56,57 Therefore, the optimized mass ratio between the battery-type electrode and capacitor-type electrode can be calculated according to the following equation:4
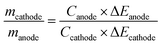 | (2) |
3. Carbon-based battery-type electrodes
3.1 Anodes
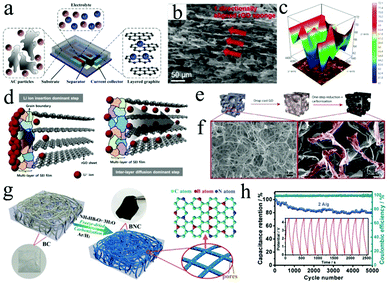 | ||
| Fig. 5 (a) Schematic of an on-chip LIC device with an AC/graphite configuration. Reproduced with permission.73 Copyright 2015, Elsevier. (b) SEM and (c) 3D mapping chemical intensity images of HOG. (d) The corresponding model of transformation kinetics in depth of lithium diffusion. Reproduced with permission.78 Copyright 2016, American Chemical Society. (e) Synthesis procedure of 3D carbon/rGO foam. (f) SEM images of neat melamine and composite foam samples. Reproduced with permission.81 Copyright 2018, Elsevier. (g) Schematic illustration of the fabrication process of BNC (B and N co-doped carbon nanofibers), and (h) corresponding cycle performance of the BNC//BNC LIC at a current density of 2 A g−1. Reproduced with permission.29 Copyright 2017, Wiley-VCH. | ||
Graphene is a single atomic plane of graphite.74 Owing to its unique two-dimensional (2D) structure, graphene displays excellent conductivity, high theoretical surface area (2630 m2 g−1) and good mechanical strength with a remarkable Young's modulus (∼1.0 TPa).75 However, the hydrophobic surface of pristine graphene sheets often leads to agglomeration in solvents. As a result, large-scale preparation of pristine graphene architectures by a solution-based process is not feasible. Therefore, graphene oxide (GO) usually acts as the precursor of graphene to rationally construct reduced graphene oxide (rGO) architectures (e.g., layered films and 3D porous architectures), that can be used as the anodes of LICs.76–81 For instance, highly oriented rGO (HOG) sponge was obtained by lyophilization and subsequent thermal reduction of GO sponge.78 The unique structure of HOG sponge could be beneficial to the fast Li ion transportation as the anodes for LICs (Fig. 5b–d). As a result, the cell based on HOG delivers excellent energy and power densities of 231.7 W h kg−1 and 57 W kg−1, respectively. In addition to the high electrochemical activity, compressible and flexible mechanical properties would also be achieved by rationally preparing the rGO-based foam electrodes (Fig. 5e and f).81 Furthermore, the conductivity of such foam can be enhanced by N doping that originates from melamine. The LIC devices based on such foam display an energy density of 40 W h kg−1, which can be maintained for 800 charge/discharge cycles. To further improve the conductivity and reduce the aggregation of rGO nanosheets, single-walled carbon nanotubes (SWCNTs) were incorporated into the rGO-based architecture.79 The LICs based on a pre-lithiated SWCNTs/rGO composite maximize the operable voltage window of 0.01–4.1 V, thus exhibiting a high energy density of 222 W h kg−1 at a power density of 410 W kg−1.
Unlike graphite and graphene, graphdiyne possesses both sp- and sp2-hybridized carbon atoms. The structure of graphdiyne is similar to the case of graphene but contains extra uniformly arranged 18C cavities, which are beneficial to the storage of Li ions. In addition, graphdiyne can be assembled into bulky architectures with a highly porous nanostructure, which can be directly used as the electrode of LICs. Their unique porous structure results in short transport pathways for ions. Li's group explored bulk graphdiyne powder as an anode material for high performance LICs. Bulk graphdiyne, featuring both micropore and mesopore morphologies, was prepared through a facile cross-coupling reaction. LICs that were constructed with a graphdiyne anode and an AC cathode exhibited higher energy and power densities, as well as excellent cyclic performance.
Compared with graphene, carbon nanotubes (CNTs) possess higher conductivity and could be assembled into freestanding porous films.82 Furthermore, functional groups can also be incorporated into CNT-based structures to enhance their performance by the synergistic effects from CNTs and functional groups.83–85 For instance, a N-doped CNT/amorphous carbon (CNT/C) composite was fabricated by carbonizing a CNT/polyaniline mixture.83 This composite possesses a mesoporous CNT architecture acting as the backbone and a thin N-rich amorphous carbon layer is coated on the CNT surface. Owing to such a unique structure, it delivers a high rate capability of 81 mA h g−1 at a high charge/discharge rate of 60C (1C = 1 h charge/discharge).
Biomass-derived carbon is usually prepared by thermally treating agricultural and forest biomass at high temperature. It is a kind of friendly electrode material due to its pollution free, large specific surface area as well as high electrochemical activity.29,86–89 For example, sisal fibers were utilized to prepare graphitic carbon through the pre-carbonization and catalytic graphitization process.89 The graphitic structure of this graphitic carbon contributes to high plateau capacity and electrical conductivity, and the porous structure supplies a smoother pathway for Li+ ion diffusion and protects the graphitic structure from etching by the electrolyte. Therefore, LICs based on graphitic carbon display a high energy density of 104 W h kg−1 at a power density of 143 W kg−1. Furthermore, heteroatom doping, which usually comes from the precursors, is also used to enhance the performance of biomass-derived carbon.29 B and N co-doped carbon nanofibers (BNC) can serve as both the anodes and cathodes of LICs due to their superior electrochemical activity in a wide voltage range (Fig. 5g). Therefore, the resultant “dual carbon” BNC//BNC LIC delivers outstanding energy (220 W h kg−1) and power densities (22.5 kW kg−1, Fig. 5h).
CNTs is one of the most common carbon materials used for supporting transition metal oxides. Moreover, CNTs are easily interconnected into a 3D conductive network, thus presenting good conductivity and flexibility.101,112,114–116 For example, nanosized TiO2 (B)/MWCNTs (multi-walled carbon nanotubes) composite films, in which TiO2 (B) nanocrystals were uniformly dispersed on a MWCNT matrix, were fabricated by an ultracentrifugation process followed by a hydrothermal treatment.107 Such a structure can effectively avoid the aggregation of TiO2 nanoparticles and enhance the power capability by enabling ultrafast Li+ de/intercalation ability (235 mA h g−1 at 100.5 A g−1) (Fig. 6a and b). Moreover, a RuO2/MWCNTs nanocomposite, where nanosized RuO2 was highly dispersed on MWCNTs, was also confirmed to be a promising anode material for LICs.117 Furthermore, the classical 1D structure of CNTs endows the composite films with the ability of acting as flexible binder-free anodes of LICs. Freestanding and flexible CNTs/transition metal oxide composite films could be prepared by different methods.96,109,118 For instance, a flexible a-Fe2O3/MWCNTs composite was prepared by a scalable spray deposition technique.118 Based on such a composite, the LIC device delivers an outstanding energy density of 50 W h kg−1 at a power density of 1000 W kg−1. This also demonstrates that the incorporation of CNTs into the transition metal oxide-based anode could lead to a decrease of internal resistance and enhancement of ion diffusion behavior of LICs.
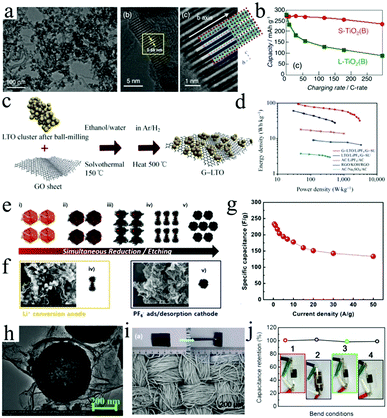 | ||
| Fig. 6 (a) SEM and HRTEM images of the (uc)-TiO2(B)/MWCNT (70/30) composite. (b) The rate capability of S-TiO2 (B) (red) and L-TiO2 (B) (green). Reproduced with permission.107 Copyright 2016, Wiley-VCH. (c) Schematic illustration of the fabrication of the G-Li4Ti5O12 nanocomposite and (d) Ragone plots for the LIC devices. Reproduced with permission.102 Copyright 2013, Springer. (e) The fabrication of graphene wrapped iron oxide (CG@SF) under HI/HCl treatment. (f) The LIC based on a CG@SF anode and a fully etched highly CG cathode. (g) Specific capacitance and rate capability of the CG@SF//CG hybrid full cell. Reproduced with permission.94 Copyright 2016, Wiley-VCH. (h) TEM image of the porous TiO2 hollow microspheres wrapped with graphene nanosheets. Reproduced with permission.113 Copyright 2016, Wiley-VCH. (i) SEM image of the Li4Ti5O12/carbon-textile electrode (inset: a digital photograph of the flexible Li4Ti5O12/CT electrode). (j) Stability of the flexible LIC measured at 0.5 A g−1 under different bending conditions (insets: digital photographs of the fully packaged LIC under different bending states). Reproduced with permission.109 Copyright 2017, Springer. | ||
Compared with CNTs, graphene possesses higher surface area, as a result, the loading of transition metal oxides on graphene will be increased.119,120 Therefore, various graphene/transition metal oxide composite architectures were designed by different strategies.121 For instance, Li4Ti5O12 nanoparticles were uniformly coated by graphene sheets through a solvothermal method.102 The assembled LIC based on this graphene/Li4Ti5O12 anode displays an ultrahigh energy density of 95 W h kg−1 at 0.4C (2.5 h, Fig. 6c and d). Different from the insertion reaction of Ti-based oxides (Table 1) and the nearly zero-strain of Li4Ti5O12, the conversion reaction of other oxides (Nb2O5, Fe2O3, SnO2) would display a faster decrease in capacity due to the severe volume changes during the charge/discharge process. Thus the fabrication of integrated graphene/metal oxide composite electrodes for LIC is highly desirable (Table 2).91,122,123 For instance, a Nb2O5/graphene composite with an orthorhombic phase, which presents outstanding pseudocapacitive behavior, was also synthesized by a simple method and further served as the anodes of LICs.122,123 Furthermore, crumpled graphene (CG) coated with spiky Fe2O3 nanoparticles (CG@SF) was prepared by combining etching and reduction processes (Fig. 6e and f).94 Since the porous particles tightly attach onto the crumpled graphene, the CG@SF offers low contact resistance and structural stability during the repetitive charge/discharge processes. Therefore, the LIC devices based on CG@SF anodes display a maximum energy density of up to 121 W h kg−1 (Fig. 6g).
| Device configuration (anode//cathode) | Voltage range | Cycling stability | Maximum energy density | Maximum power density | Ref. |
|---|---|---|---|---|---|
| TiO2@mesoporous carbon//AC | 0–3.0 V | 80.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
67.4 W h kg−1 | 5000 W kg−1 | 108 |
| TiO2 hollow spheres@graphene//graphene | 0–3.0 V | 65% after 1000 cycles | 72 W h kg−1 | 2000 W kg−1 | 113 |
| TiO2 nanobelt//graphene hydrogels | 0–3.8 V | 73% after 600 cycles | 82 W h kg−1 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
126 |
| TiO2@rGO//AC | 1.0–3.0 V | — | 42 W h kg−1 | 8000 W kg−1 | 90 |
| H-TiO2/PPy/SWCNTs//AC | 1.0–3.0 V | ∼77.8% after 3000 cycles | 31.3 W h kg−1 | 4000 W kg−1 | 112 |
| TiO2-CNT//activated carbon | 1.0–3.0 V | ∼ | 59.6 W h kg−1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 W kg−1 900 W kg−1 |
127 |
| Li4Ti5O12/CNT//AC | 1.5–2.8 V | 92% after 6000 cycles | 84.2 W h kg−1 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652.5 W kg−1 652.5 W kg−1 |
128 |
| Li4Ti5O12 nanowires//AC | 0–2.5 V | 84% after 1000 cycles | 18.44 μW h cm−2 | — | 106 |
| 100-Li4Ti5O12-G-600C//AC | 1.5–3.0 V | 97% after 2000 cycles | 52 W h kg−1 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 W kg−1 400 W kg−1 |
129 |
| Li4Ti5O12/CT//NGC | 1.0–3.0 V | 79% after 5000 cycles | 2 mW h cm−3 | 185 mW cm−3 | 109 |
| Li4Ti5O12–graphene//AC | 1–2.5 V | — | 30 W h kg−1 | 1000 W kg−1 | 104 |
| TiO2-coated Li4Ti5O12//AC | 0.5–2.5 V | 83% after 5000 cycles | 74.85 W h kg−1 | 7500 W kg−1 | 130 |
| Graphene-wrapped Li4Ti5O12//AC | 1.0–2.5 V | 75% after 1000 cycles | 50 W h kg−1 | 2500 W kg−1 | 131 |
| Spheres Li4Ti5O12//AC | 1.0–3.5 V | 93% after 500 cycles | 74.3 W h kg−1 | 468.7 W kg−1 | 132 |
| Li4Ti5O12//N-doped porous carbon | 1.0–3.0 V | — | 63 W h kg−1 | 5000 W kg−1 | 133 |
| Graphene-Li4Ti5O12//graphene-sucrose | 0–3.0 V | 94% after 500 cycles | 95 W h kg−1 | 3000 W kg−1 | 102 |
| Device configuration (anode//cathode) | Voltage range | Cycling stability | Maximum energy density | Maximum power density | Ref. |
|---|---|---|---|---|---|
| Nb2O5 film//AC | 1.0–3.5 V | 87% after 1000 cycles | 95.55 W h kg−1 | 5350.9 W kg−1 | 93 |
| Nb2O5-carbide-derived carbon//AC | 1.0–2.8 V | — | 30 W h kg−1 | 5000 W kg−1 | 144 |
| T-Nb2O5@C//AC | 1.0–3.5 V | 75% after 1000 cycles | 63 W h kg−1 | 6500 W kg−1 | 145 |
| Mesoporous Nb2O5–C//AC | 1.0–3.5 V | — | 74 W h kg−1 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 W kg−1 137 W kg−1 |
146 |
| T-Nb2O5–graphene//AC | 0.8–3 V | 93% after 2000 cycles | 47 W h kg−1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
147 |
| Nb2O5–CNT//AC | 0.5–3 V | — | 33.5 W h kg−1 | 4000 W kg−1 | 148 |
| Graphene wrapped Fe2O3//graphene | 1.0–4.0 | 87% after 2000 cycles | 121 W h kg−1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
94 |
| Fe2O3@C//N-HPC | 1.0–4.0 V | 84.1% after 1000 cycles | 65 W h kg−1 | 9200 W kg−1 | 149 |
| FexO@graphene//porous graphene | 0–3.5 V | 75% after 3000 cycles | 129.6 W h kg−1 | 3500 W kg−1 | 150 |
| Fe2O3//AC | 0–3.5 V | 55% after 2500 cycles | 90 W h kg−1 | — | 151 |
| Fe3O4 in graphene//3D graphene | 1.0–4.0 V | 70% after 1000 cycles | 204 W h kg−1 | 2650 W kg−1 | 152 |
| AC:SnO2/Cu/CNT//AC | — | 81% after 200 cycles | 90 W h kg−1 | — | 116 |
Significantly, the composites of carbon and metal oxides can not only fabricate traditional-shaped LICs, but also construct fiber-shaped LICs. For example, lightweight and portable fiber-shaped LICs were designed by using Li4Ti5O12 as anodes and AC as cathodes.106 This research would give more suggestions to develop fiber-shaped energy storage devices with high electrochemical performance. To further improve the safety of LICs, quasi-solid-state LICs based on TiO2@graphene anodes were also fabricated (Fig. 6h).113 Such quasi-solid-state LICs can achieve a maximum energy density of 72 W h kg−1 based on the total mass of electrode materials. Furthermore, graphene encapsulated Fe3O4 cube composite films (rGO@Fe3O4) were also fabricated as the anodes of flexible quasi-solid-state LICs.134 In this composite, ultrathin and flexible graphene shells uniformly enwrapped the Fe3O4 nanocube, which could effectively accommodate the volume variation of Fe3O4 and also suppress the aggregation of Fe3O4 during the cycling process. In addition to CNTs or graphene, there are still many carbon materials that can be used as conductive/supportive agents, such as AC,135 PPy,112 CMK-3,136 carbon nanofibers,96 carbon cloth,93 and so on. For example, Li4Ti5O12 nanoplates were coated on carbon textile (LTO/CT) to serve as the anode of flexible LICs.109 Owing to the excellent mechanical strength of LTO/CT composite films (Fig. 6i), the flexible LICs present extremely small capacity fluctuation under different bending states (Fig. 6j). Recently, Jiang et al. found that Li4Ti5O12/AC composite anodes present increased capacity retention as the AC content increases in the composite even though their overall specific capacity decreases gradually.135 These results demonstrate that it is of great significance to improve the electrochemical properties and mechanical strength of metal oxide-based anodes by incorporating carbon materials into the composite anodes.
Compared to transition metal oxides, transition metal carbonitride has excellent chemical stability, good electrical conductivity and outstanding oxidation/corrosion resistance (Table 3).137–139 Owing to the high theoretical specific capacity (∼1043 mA h g−1) and obvious pseudocapacitive characteristics, VN is a popular candidate for anodes of LICs.124,140 Nevertheless, pure VN nanowires deliver only a capacity of 400 mA h g−1 at 0.1 A g−1 and thus their charge storage ability has to be improved for fabricating LICs with high performance. Fortunately, creating a 3D porous structure that combines VN nanowires with graphene nanosheets could effectively enhance the charge-storage ability because of the formation of abundant pore channels (Fig. 7a and b).124 As a result, VN–RGO//carbon-based LIC devices exhibit a high energy density of ∼162 W h kg−1 at a power density of 200 W kg−1 as well as a high power density of 10 kW kg−1 at an energy density of ∼64 W h kg−1 (Fig. 7c). Similarly, graphene can also be added to a NbN-based electrode to further enhance the charge storage ability of Li+ ions in NbN. A NbN/N-doped graphene nanocomposite was controllably prepared by combining a simple hydrothermal process with ammonia annealing (Fig. 7d).125 The fabricated nanocomposite delivers a high energy density of 122.7–98.4 W h kg−1 at 100–2000 W kg−1 (Fig. 7e and f). The enhanced electrochemical performance is mainly due to the size of the as-obtained NbN nanoparticles of about 10–15 nm and the addition of graphene nanosheets, whose structure could improve the utilization of active materials and relieve the aggregation of nanoparticles. Importantly, owing to the flexibility of graphene, a freestanding layer-stacked composite architecture, integrating 0D NbN nanoparticles with 2D graphene nanosheets (GNS), was also fabricated.154 Based on the free-standing NbN/GNSs anode, the LIC delivers outstanding energy (136 W h kg−1) and power (25 kW kg−1) densities in the voltage range of 1.0–4.0 V. Similar to nitrides, carbides, such as TiC, also have excellent chemical stability and good electrical conductivity. Fan's group rationally designed a TiC nanoparticle chain anode by a carbothermal conversion of graphene/TiO2 hybrid aerogels with a 3D conductive network.138 Owing to the unique structure of the TiC electrode, the assembled PHPNC//TiC LIC shows excellent potential for bridging-the-gap between conventional LIBs and SCs.
| Device configuration (anode//cathode) | Voltage range | Cycling stability | Maximum energy density | Maximum power density | Ref. |
|---|---|---|---|---|---|
| CTAB–Sn(IV)@Ti3C2//AC | 1.0–4.0 | 71% after 4000 cycles | 239.50 W h kg−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 W kg−1 800 W kg−1 |
139 |
| TiC//N-doped porous carbon | 0–4.5 V | 82% after 5000 cycles | 101.5 W h kg−1 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 W kg−1 500 W kg−1 |
138 |
| VN–rGO//activated carbon | 0–4 V | 83% after 1000 cycles | 162 W h kg−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
124 |
| NbN/nitrogen-doped graphene//AC | 2.0–4.0 V | 81% after 1000 cycles | 122.7 W h kg−1 | 2000 W kg−1 | 125 |
| NbN//PANI-derived carbon | 0–4 V | 95% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
149 W h kg−1 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
153 |
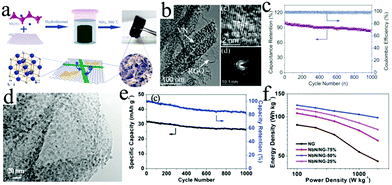 | ||
| Fig. 7 (a) Schematic of the fabrication of a 3D VN–RGO composite. (b) TEM, HRTEM and selected area electron diffraction (SAED) patterns of the 3D VN–RGO composite. (c) Cycle stability for 1000 cycles at a current density of 2 A g−1. Reproduced with permission.124 Copyright 2015, Wiley-VCH. (d) Low-magnification TEM image of the NbN/NG-50% hybrid material. (e) Cycle performance of the NbN/NG-50% based LIC at 500 mA g−1, and (f) Ragone plot of NG and NbN/NG-X based LICs (X = 75%, 50%, 25%). Reproduced with permission.125 Copyright 2015, Wiley-VCH. | ||
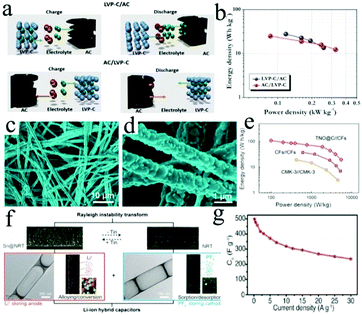 | ||
| Fig. 8 (a) Schematic representation of the charge storage mechanism of LICs based on LVP-C/AC and AC/LVP-C. (b) Ragone plots showing the performance of LVP-C/AC and AC/LVP-C. Reproduced with permission.141 Copyright 2015, Elsevier. (c and d) SEM images of the TiNb2O7 MWs. (e) Ragone plots of the TNO@C//CFs LIC, CFs//CFs and CMK-3//CMK-3 symmetric SCs. Reproduced with permission.142 Copyright 2015, Elsevier. (f) Morphology of the Sn@NRT (N-rich nanotube) and the NRT structure with illustration showing the ion penetration process. (g) The rate capability of the Sn@NRT||NRT LIC at different current densities. Reproduced with permission.143 Copyright 2017, Wiley-VCH. | ||
TiNb2O7 can act as an alternative candidate for Li4Ti5O12 as the anodes of LICs. The monoclinic crystal structure of TiNb2O7 is formed by disordered Nb and Ti atoms, which could provide two dimensional interstitial spaces for fast Li ion insertion. TiNb2O7@C microwires were synthesized by an electrospinning method, followed by carbon coating at high temperature (Fig. 8c and d).142 The as-fabricated LICs based on TiNb2O7@C//CFs exhibit an energy density of 110.4 W h kg−1 at 99.58 W kg−1 (Fig. 8e). Furthermore, a TiNb2O7/holey graphene (TNO/HG) nanocomposite with superior rate capability and long-term cycling performance was prepared through in situ anchoring of a TiNb2O7 network nanostructure onto 2D holey graphene.157 Importantly, the Li insertion behavior of the TiNb2O7@C electrode was studied in depth and the intercalation pseudo-capacitive reaction mechanism was achieved.
In addition to the above mentioned materials, nano-TiP2O7,158 NASICON-type LiTi1.5Zr0.5(PO4)159 and LiSn2(PO4)3160 were also reported for application in LICs. Because they were not composites with carbon, we cannot review those materials in detail.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 6.4 A g−1. Besides, heteroatom B-doping is also a facile way to modify Si electrodes. For instance, B-Si/SiO2/C used as the anode of LICs delivers a high energy density of 128 W h kg−1 at 1229 W kg−1 and a high power density of 9704 W kg−1 at 89 W h kg−1.165 To further improve the safety of LICs, a solid-state LIC was fabricated using a Si-VACNFs (vertically aligned carbon nanofibers) anode in conjunction with a flexible, solid-like gel polymer film as the electrolyte and separator.167 Due to the synergistic effect of Si-VACNFs and gel polymer, the LIC could stably operate in the temperature range from −20 to 60 °C with a very low self-discharge rate and an excellent shelf life.
000 cycles at 6.4 A g−1. Besides, heteroatom B-doping is also a facile way to modify Si electrodes. For instance, B-Si/SiO2/C used as the anode of LICs delivers a high energy density of 128 W h kg−1 at 1229 W kg−1 and a high power density of 9704 W kg−1 at 89 W h kg−1.165 To further improve the safety of LICs, a solid-state LIC was fabricated using a Si-VACNFs (vertically aligned carbon nanofibers) anode in conjunction with a flexible, solid-like gel polymer film as the electrolyte and separator.167 Due to the synergistic effect of Si-VACNFs and gel polymer, the LIC could stably operate in the temperature range from −20 to 60 °C with a very low self-discharge rate and an excellent shelf life.
Compared to Si, Sn has a superior electronic conductivity, leading to the fact that Sn anodes would display a better rate capability. Nevertheless, Sn also suffers from a large volume change during the charge/discharge process, which can result in severe polarization of the electrode material.168–170 Currently, the design of small Sn nanoparticles with a coupling carbon substrate is an effective approach to overcome the above issues. For example, a Sn-C nanocomposite was synthesized by a facile confined growth method,171 and the composite possessed multiple structural advantages including well-dispersed ultrafine Sn nanoparticles, an interconnected spherical network and a large specific surface area. As a result, the LICs exhibit high power densities of 731.25 W kg−1 and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 W kg−1 at energy densities of 195.7 W h kg−1 and 84.6 W h kg−1, respectively. To further motivate the ion diffusion kinetics and accommodate Sn nanoparticle aggregation, Kang's group143 fabricated an Sn@NRT (N-rich nanotube) anode with open mesoporous channels, where the ultrasmall Sn particles (∼3.8 nm) were embedded in the 1D CNTs (Fig. 8f). Indeed, the LIC device based on the Sn@NRT||NRT anode was found to exhibit a high energy density of 274 W h kg−1 at 153 W kg−1 and a high power density of 22
375 W kg−1 at energy densities of 195.7 W h kg−1 and 84.6 W h kg−1, respectively. To further motivate the ion diffusion kinetics and accommodate Sn nanoparticle aggregation, Kang's group143 fabricated an Sn@NRT (N-rich nanotube) anode with open mesoporous channels, where the ultrasmall Sn particles (∼3.8 nm) were embedded in the 1D CNTs (Fig. 8f). Indeed, the LIC device based on the Sn@NRT||NRT anode was found to exhibit a high energy density of 274 W h kg−1 at 153 W kg−1 and a high power density of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 W kg−1 at 127 W h kg−1 (Fig. 8g).
800 W kg−1 at 127 W h kg−1 (Fig. 8g).
3.2 Pre-lithiation
Pre-lithiation is usually used in a system with neither electrode containing lithium atoms.54 Such a procedure is an effective strategy to reduce the potential of the anode to bring it close to that of Li/Li+ and to compensate for the ion loss during solid electrolyte interface/cathode electrolyte interface layer formation. The former one can broaden the working voltage and improve the energy density of LICs, while the latter one can avoid the irreversible capacity loss and improve the cycling stability of LICs.45,172 Significantly, pre-lithiation is also a popular technique to increase the Li+ concentration in the electrolyte.The most popular method for pre-lithiation is an electrochemical strategy. Similar to the assembly method of a half-cell, such a method through the direct electrochemical charge/discharge process achieves Li+ ion doping in the anode material.173 The primary merit of such an approach is the good controllability of applying a programmable procedure. Nevertheless, the assembly/disassembly cell process could limit its large scale application in LICs.50 Compared to the electrochemical strategy, a chemical charging method, also called an external short circuit, is a more scalable approach via a chemical reaction between the lithium metal and the electrode material in the Li-containing electrolyte.47,174 However, the amount of Li ions embedded in electrode material cannot be understand clearly during the pre-lithiation process, since it is impossible to control or monitor the state of electric charge.
Besides, direct contact between the active material and metal Li is also used to realize Li+ doping.44,46 Park et al. studied the lithiation process of graphite anodes via the direct contact method using in situ synchrotron wide-angle X-ray scattering for LICs.71 But the uncertainty of the amount of pre-embedded lithium is still a challenge for the direct contact method. Although pre-lithiation has certain merits while applying to LIC systems, some of the issues caused by pre-lithiation cannot be ignored.46 For instance, SEI formation results in an increase in the internal resistance, thus hindering the attainment of a higher power density.
3.3 Cathodes
4. Carbon-based capacitor-type electrodes
4.1 Cathodes
| Device configuration (anode//cathode) | Voltage range | Cycling stability | Maximum energy density | Maximum power density | Ref. |
|---|---|---|---|---|---|
| ANCS//ANCS | 0–4.5 V | 88% after 9000 cycles | 206.7 W h kg−1 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 W kg−1 500 W kg−1 |
30 |
| N-Doped carbon nanopipes//reduced graphene oxides | 0–4.0 V | 91% after 4000 cycles | 262 W h kg−1 | 9000 W kg−1 | 189 |
| BNC//BNC | 0–4.5 V | 81% after 5000 cycles | 220 W h kg−1 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 W kg−1 500 W kg−1 |
29 |
| Hard carbon//bio-derived mesoporous carbon | 1.7–4.2 V | 81% after 8000 cycles | 121 W h kg−1 | 9000 W kg−1 | 88 |
| Graphdiyne//AC | 2.0–4.0 V | 94.7% after 1000 cycles | 110.7 W h kg−1 | 1000.35 W kg−1 | 187 |
| Graphene//armored graphene | 0–4.3 V | 89% after 1000 cycles | 160 W h kg−1 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
190 |
| Oriented rGO sponge//AC | 1.5–4.0 V | 84.2% after 1000 cycles | 231.7 W h kg−1 | 2800 W kg−1 | 78 |
| Reduced GO//resin-derived carbon combined with GO | 0–4.0 V | 79% after 3000 cycles | 148.3 W h kg−1 | 6500 W kg−1 | 77 |
| Microcrystalline graphite//mesoporous carbon nanospheres/graphene | 2.2–4.2 V | 93% after 4000 cycles | 80 W h kg−1 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 W kg−1 600 W kg−1 |
191 |
| N-Doped hard carbon//activated carbon | 2.0–4.0 V | 97% after 5000 cycles | 28.5 W h kg−1 | 6940 W kg−1 | 192 |
| Graphene//activated carbon | 2.0–4.0 V | 74% after 300 cycles | 95 W h kg−1 | 222.2 W kg−1 | 193 |
| Graphite//graphene | 2.0–4.0 V | 97% after 3500 cycles | 135 W h kg−1 | 1500 W kg−1 | 194 |
| Soft carbon//activated carbon | 0–4.4 V | 63% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
115 W h kg−1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
195 |
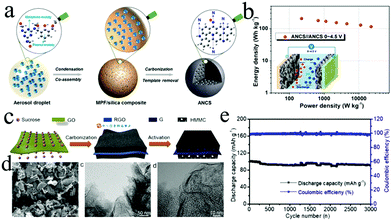 | ||
| Fig. 9 (a) Schematic illustration of the ANCS formation process. (b) Ragone plots of a LIC based on the bifunctional ANCS electrode. Reproduced with permission.30 Copyright 2018, American Chemical Society. (c) The fabrication process of the G@HMMC material. (d) The SEM and TEM images of the G@HMMC850 material. (e) Long cycling performance of a LIC composed of G@HMMC850 and pre-lithiated graphite. Reproduced with permission.184 Copyright 2018, Elsevier. | ||
Owing to the unsatisfactory specific capacitance of commercial AC, many researchers have shifted attention to various biomass-derived AC materials recently. Biomass-derived carbon can exhibit nanotubular, nanofibrillar, and lamellar structures. Importantly, the hierarchical pore structure of biomass-derived carbon is easy to achieve during the high-temperature calcination process, which is beneficial to electrolyte infiltration. The advantages of the controllable microstructure and tunable surface functional groups make the biomass-derived AC materials more attractive than commercial AC for LICs.59 For instance, the AC derived from egg white shows a high surface area of 3250 m2 g−1 with micropore size from 0.8 to 1.4 nm, which contributes to the high capacitance and excellent rate performance in LICs.86 As a result, the optimized LICs based on this AC cathode deliver a high energy density of 257 W h kg−1 at 867 W kg−1. Recently, hierarchical porous carbon with a larger specific surface area of 3898 m2 g−1 was rationally synthesized by using egg white biomass as a precursor and NaCl as a template, and was then used as the cathodes of LICs.196 In general, owing to the complexity of components, biomass-derived carbon is usually doped with heteroatoms, such as S, N, P and so on. Heteroatom doping can effectively change the electron distribution of carbon materials, which can improve the electron conductivity of active materials. Furthermore, surface functionalization can further improve the capacitive performance of carbon materials since doping can lead to redox reactions, electron donor capability, or/and electrode wettability. For example, N-doped AC (NAC)86 with a moderate N content of 4 wt% was prepared utilizing agricultural waste (corncob) as a precursor.197,198 The obtained AC with a narrow micro-/meso-pore distribution has a high specific surface area of up to 2859 m2 g−1. The LIC using NAC as the cathode shows an energy density as high as 230 W h kg−1 at a power density of 1747 W kg−1. Moreover, coatings with non-carbon films to enhance the electrochemical performance of carbon materials have recently begun to gain attention. For instance, a thin TiO2/C composite film coated on porous biomass-derived biocarbon (PBC) (denoted as PBC@TC) was fabricated by a facile sol–gel strategy.199 The results show a distinct Li+ storage capacity improvement for the PBC@TC electrode compared with the uncoated electrode, demonstrating the significant positive effect of TiO2/C ultrathin-films on improving the electrochemical performance of the carbon matrix. However, high temperature calcination could lead to high energy consumption, which would increase the production cost.
In addition, metal organic framework (MOF)-derived carbon with various architectures has also been widely used in LICs. For example, high surface area (2714 m2 g−1) carbon cuboids were synthesized by pyrolysing the zinc based MOF-5, which exhibits a unique crumpled-sheet assembled porous morphology with the desired levels of micro and mesoporosity.200 Based on the advanced structure, the MOF-based LIC delivers a maximum specific energy density of 65 W h kg−1 with excellent power capability. Similarly, MOF-derived polyhedral hollow carbon was also fabricated and used in LICs.201 It is worth mentioning that removal of metal ions is a necessary process during the preparation of MOF-derived carbon due to the existence of metal ions in the MOF precursor.
The unique 2D structure and physical properties of graphene and its derivatives make them distinctive building blocks for fabricating various 3D porous architectures. Such 3D architectures exhibit excellent chemical stability and high specific surface area as well as fast electron transport kinetics due to the combination of porous structures and the excellent intrinsic properties of graphene and its derivatives. Thus, these 3D architectures will be the promising cathodes of LICs with high performance. Various strategies have been reported to construct graphene-based architectures for LICs.202 For example, micron-sized porous graphene belts are fabricated by a chemical vapor deposition (CVD) approach.203 Owing to the high ratio of length to diameter (up to 100), good structural integrity and few-layered structure, the obtained graphene belts exhibit good durability and satisfactory Ragone performance (8044 W kg−1 at 51 W h kg−1 and 120 W h kg−1 at 503 W kg−1). Moreover, activated and expanded graphite oxide was synthesized by a micro-wave exfoliation method.53 Using such activated graphite oxide as the cathode, the packaged LIC delivers a gravimetric energy density of 53.2 W h kg−1 at an operating voltage of 4 V. Importantly, the incorporation of microporous carbon with surface functionalization into graphene architectures is an effective strategy to further improve the power and energy densities of LICs. A graphene@meso-/microporous carbon with rational oxygen containing groups was prepared by combining carbonization and activation processes.184 In the rationally designed G@HMMC electrode (Fig. 9c and d), the hierarchical meso-/microporous carbon offers abundant active sites and the graphene network provides more channels for electrons. Therefore, the LIC with the G@HMMC cathode displays ultrahigh energy densities of 233.3–143.8 W h kg−1 at 450.4 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 686 W kg−1 (Fig. 9e).204
686 W kg−1 (Fig. 9e).204
Owing to their unique hollow architecture, appropriate pore size and ability to form nanoscale network structures, CNTs are considered as one of the most popular cathode materials for LICs. A flexible nanostructured LIC using thin film MWCNTs as the cathode and a-Fe2O3/MWCNT composite as the anode was fabricated.118 On the basis of the total weight of the anode and cathode, the LIC displays a very high specific energy density of 50 W h kg−1 at a specific power density of 1000 W kg−1 in the voltage range of 0–2.8 V. In cathodes based on CNTs, the good electrical conductivity of the MWCNTs is beneficial to improve Li-ion transport kinetics and cycling performance. Although CNTs have good rate characteristics, the complex purification processes and high production costs would hinder their practical application. Similar to CNTs, one-dimensional carbon nanofibers possess superior kinetic properties due to their high aspect ratio and oriented electronic/ionic transport paths, making them promising candidates for LIC electrodes. Shi et al. constructed a highly graphitized carbon fiber with a hierarchical porous structure by electrospinning followed by carbonization and chemical activation.205 Owing to the synergistic effect of large specific surface area (up to 2157.4 m2 g−1), hierarchical porous structure, as well as greatly improved electrical conductivity, the as-assembled LIC with carbon fiber cathode and Fe3O4 anode delivers a maximum energy density of 124.6 W h kg−1 with excellent rate capability, demonstrating a promising approach for developing advanced activated carbon electrodes for high-performance LICs.
5. Conclusion and perspective
The progress of LICs is mostly benefited from the development of advanced carbon-based electrodes. Based on diverse carbon-based materials, LIC devices with high energy and power densities were designed and assembled. This review summarizes recent developments of carbon-based materials as the anodes and cathodes of LICs. The carbon-based anode materials focus on carbonaceous materials, carbon/transition metal compounds, carbon/polyanion composites, carbon/metalloid and metal materials. In addition to anodes, carbon-based materials also play an important role in the design of cathodes of LICs, where carbonaceous materials and carbon/metal compound composites are highlighted.Although great efforts have been devoted to the fabrication of carbon-based electrodes and the design of LICs based on them, much work still remains to be done. The inherent electrochemical performance of the carbon materials and the growth of active materials on the carbon materials depend on the porous structure and the surface properties of carbon materials. Various carbon materials have been developed to serve as the electrodes directly or to support other active materials. However, in most cases, their microstructures are disordered and random and their surface properties are not controlled well. Furthermore, some pores of these materials are not electrochemically accessible during the contact with the electrolyte, limiting the electrochemical performance of carbon-based electrodes. Thus rational design of carbon materials with precisely controllable and novel microstructures, sizes, shapes, and surface area should be further considered. Besides, the morphology, distribution and mass loading of active materials on carbon materials could be rationally controlled to enhance the synergistic effects between active materials and carbon materials.
The recent boom in electronic devices with different functions has increased the demand for flexible energy storage devices. The design of flexible LICs mainly depends on the fabrication of cathodes and anodes with both electrochemical and mechanical properties. Although some flexible cathodes and anodes have been achieved based on carbon materials such as CNTs and graphene by different methods, the specific surface area, conductivity, and mechanical properties of CNTs and graphene are not fully utilized in these flexible electrodes. As a result, the electrochemical properties of LICs based on them often degrade under repeatedly bending states. Therefore, cathodes and anodes with enhanced electrochemical and mechanical properties should be further developed for practical applications. Besides LICs, such guidance can also be applied to sodium-ion hybrid capacitors, Li–S hybrid capacitors, magnesium ion hybrid capacitors, lithium–air capacitor-battery and so on. Carbon-based materials will continue to drive the innovation in science and technology in the field of LICs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21573116, 51822205, 21875121 and 51602218), Ministry of Science and Technology of China (2017YFA0206701), Ministry of Education of China (B12015), Tianjin Basic and High-Tech Development (18JCQNJC02400), the Fundamental Research Funds for the Central Universities and the Young Thousand Talents Program.Notes and references
- Y. Da, J. Liu, L. Zhou, X. Zhu, X. Chen and L. Fu, Adv. Mater., 2019, 31, 1802793 CrossRef PubMed.
- C. Chen, X. Xie, B. Anasori, A. Sarycheva, T. Makaryan, M. Zhao, P. Urbankowski, L. Miao, J. Jiang and Y. Gogotsi, Angew. Chem., Int. Ed., 2018, 57, 1846–1850 CrossRef CAS PubMed.
- M. R. Lukatskaya, B. Dunn and Y. Gogotsi, Nat. Commun., 2016, 7, 12647 CrossRef PubMed.
- P. Jeżowski, O. Crosnier, E. Deunf, P. Poizot, F. Béguin and T. Brousse, Nat. Mater., 2018, 17, 167 CrossRef PubMed.
- S. Guo, H. Li, Y. Li, Y. Han, K. Chen, G. Xu, Y. Zhu and X. Hu, Adv. Energy Mater., 2018, 8, 1800434 CrossRef.
- Z. Liu, S. Yang, B. Sun, X. Chang, J. Zheng and X. Li, Angew. Chem., Int. Ed., 2018, 57, 10187–10191 CrossRef CAS PubMed.
- X. Ma, J. Cheng, L. Dong, W. Liu, J. Mou, L. Zhao, J. Wang, D. Ren, J. Wu, C. Xu and F. Kang, Energy Storage Mater., 2018 DOI:10.1016/j.ensm.2018.10.020.
- P. Li, Z. Jin, L. Peng, F. Zhao, D. Xiao, Y. Jin and G. Yu, Adv. Mater., 2018, 30, e1800124 CrossRef PubMed.
- P. Yang, Z. Wu, Y. Jiang, Z. Pan, W. Tian, L. Jiang and L. Hu, Adv. Energy Mater., 2018, 8, 1801392 CrossRef.
- H. Li, J. Lang, S. Lei, J. Chen, K. Wang, L. Liu, T. Zhang, W. Liu and X. Yan, Adv. Funct. Mater., 2018, 28, 1800757 CrossRef.
- C. Wang, H. Xie, S. Chen, B. Ge, D. Liu, C. Wu, W. Xu, W. Chu, G. Babu, P. M. Ajayan and L. Song, Adv. Mater., 2018, 30, e1802525 CrossRef PubMed.
- B. Li, J. Zheng, H. Zhang, L. Jin, D. Yang, H. Lv, C. Shen, A. Shellikeri, Y. Zheng, R. Gong, J. P. Zheng and C. Zhang, Adv. Mater., 2018, 30, e1705670 CrossRef PubMed.
- J. Ding, W. Hu, E. Paek and D. Mitlin, Chem. Rev., 2018, 118, 6457–6498 CrossRef CAS PubMed.
- K. Lu, D. Li, X. Gao, H. Dai, N. Wang and H. Ma, J. Mater. Chem. A, 2015, 3, 16013–16019 RSC.
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC.
- H. Wang, C. Zhu, D. Chao, Q. Yan and H. J. Fan, Adv. Mater., 2017, 29, 1702093 CrossRef PubMed.
- X. Y. Yu and X. W. David Lou, Adv. Energy Mater., 2018, 8, 1701592 CrossRef.
- G. Yu, X. Xie, L. Pan, Z. Bao and Y. Cui, Nano Energy, 2013, 2, 213–234 CrossRef CAS.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017, 4, 1600539 CrossRef PubMed.
- J. Zhang, W. Lv, D. Zheng, Q. Liang, D.-W. Wang, F. Kang and Q.-H. Yang, Adv. Energy Mater., 2018, 8, 1702395 CrossRef.
- Z. Wu, L. Li, J. M. Yan and X. B. Zhang, Adv. Sci., 2017, 4, 1600382 CrossRef PubMed.
- X. Wang, K. Cao, Y. Wang and L. Jiao, Small, 2017, 13, 1700873 CrossRef PubMed.
- H. Gu, Y.-E. Zhu, J. Yang, J. Wei and Z. Zhou, ChemNanoMat, 2016, 2, 578–587 CrossRef CAS.
- J. Lang, X. Zhang, B. Liu, R. Wang, J. Chen and X. Yan, J. Energy Chem., 2018, 27, 43–56 CrossRef.
- L. Wang and X. Hu, Chem. – Asian J., 2018, 13, 1518–1529 CrossRef CAS PubMed.
- L. Dong, X. Ma, Y. Li, L. Zhao, W. Liu, J. Cheng, C. Xu, B. Li, Q.-H. Yang and F. Kang, Energy Storage Mater., 2018, 13, 96–102 CrossRef.
- P. Han, G. Xu, X. Han, J. Zhao, X. Zhou and G. Cui, Adv. Energy Mater., 2018, 8, 1801243 CrossRef.
- B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang and M. Cai, Adv. Energy Mater., 2016, 6, 1600802 CrossRef.
- Q. Xia, H. Yang, M. Wang, M. Yang, Q. Guo, L. Wan, H. Xia and Y. Yu, Adv. Energy Mater., 2017, 7, 1701336 CrossRef.
- F. Sun, X. Liu, H. B. Wu, L. Wang, J. Gao, H. Li and Y. Lu, Nano Lett., 2018, 18, 3368–3376 CrossRef CAS PubMed.
- J. Yang, C. Yu, C. Hu, M. Wang, S. Li, H. Huang, K. Bustillo, X. Han, C. Zhao, W. Guo, Z. Zeng, H. Zheng and J. Qiu, Adv. Funct. Mater., 2018, 28, 1803272 CrossRef.
- Y.-J. Heo, J. W. Lee, Y.-R. Son, J.-H. Lee, C. S. Yeo, T. D. Lam, S. Y. Park, S.-J. Park, L. H. Sinh and M. K. Shin, Adv. Energy Mater., 2018, 8, 1801854 CrossRef.
- L. Fan, K. Lin, J. Wang, R. Ma and B. Lu, Adv. Mater., 2018, 30, e1800804 CrossRef PubMed.
- G. G. Amatucci, F. Badway, A. Du Pasquier and T. Zheng, J. Electrochem. Soc., 2001, 148, A930 CrossRef CAS.
- Y. Ma, H. Chang, M. Zhang and Y. Chen, Adv. Mater., 2015, 27, 5296–5308 CrossRef CAS PubMed.
- V. Aravindan, J. Gnanaraj, Y. S. Lee and S. Madhavi, Chem. Rev., 2014, 114, 11619–11635 CrossRef CAS.
- K. Naoi, Fuel Cells, 2010, 10, 825–833 CrossRef CAS.
- F. Yao, D. T. Pham and Y. H. Lee, ChemSusChem, 2015, 8, 2284–2311 CrossRef CAS.
- J. Balamurugan, T. T. Nguyen, V. Aravindan, N. H. Kim and J. H. Lee, Adv. Funct. Mater., 2018, 1804663, DOI:10.1002/adfm.201804663.
- Q. Rong, W. Lei, J. Huang and M. Liu, Adv. Energy Mater., 2018, 8, 1801967 CrossRef.
- K. Naoi, S. Ishimoto, J.-I. Miyamoto and W. Naoi, Energy Environ. Sci., 2012, 5, 9363 RSC.
- H. Shang, Z. Zuo, L. Yu, F. Wang, F. He and Y. Li, Adv. Mater., 2018, 30, e1801459 CrossRef PubMed.
- J. P. Zheng, J. Electrochem. Soc., 2009, 156, A500 CrossRef CAS.
- A. Schiele, B. Breitung, T. Hatsukade, B. B. Berkes, P. Hartmann, J. Janek and T. Brezesinski, ACS Energy Lett., 2017, 2, 2228–2233 CrossRef CAS.
- R. Tian, H. Duan, Y. Guo, H. Li and H. Liu, Small, 2018, 14, 1802226 CrossRef PubMed.
- L. Shi, C. Pang, S. Chen, M. Wang, K. Wang, Z. Tan, P. Gao, J. Ren, Y. Huang, H. Peng and Z. Liu, Nano Lett., 2017, 17, 3681–3687 CrossRef CAS PubMed.
- Y. Jin, S. Li, A. Kushima, X. Zheng, Y. Sun, J. Xie, J. Sun, W. Xue, G. Zhou, J. Wu, F. Shi, R. Zhang, Z. Zhu, K. So, Y. Cui and J. Li, Energy Environ. Sci., 2017, 10, 580 RSC.
- J. Wang, Y. Cui and D. Wang, Adv. Mater., 2018, 30, 1801993 CrossRef PubMed.
- B. Li, S.-W. Chien, X. Ge, J. Chai, X.-Y. Goh, K.-T. Nai, T. S. Andy Hor, Z. Liu and Y. Zong, Mater. Chem. Front., 2017, 1, 677–682 RSC.
- Q. Xu, J.-K. Sun, G. Li, J.-Y. Li, Y.-X. Yin and Y.-G. Guo, Chem. Commun., 2017, 53, 12080 RSC.
- X. Wang, Y. Liu, Y. Wang and L. Jiao, Small, 2016, 12, 4865–4872 CrossRef CAS PubMed.
- Y. Liu, N. Zhang, C. Yu, L. Jiao and J. Chen, Nano Lett., 2016, 16, 3321–3328 CrossRef CAS PubMed.
- M. D. Stoller, S. Murali, N. Quarles, Y. Zhu, J. R. Potts, X. Zhu, H. W. Ha and R. S. Ruoff, Phys. Chem. Chem. Phys., 2012, 14, 3388–3391 RSC.
- P. V. Braun and J. B. Cook, ACS Nano, 2018, 12, 3060–3064 CrossRef CAS PubMed.
- J. Zhang, D.-W. Wang, W. Lv, L. Qin, S. Niu, S. Zhang, T. Cao, F. Kang and Q.-H. Yang, Adv. Energy Mater., 2018, 8, 1801361 CrossRef.
- V. Augustyn, J. Come, M. A. Lowe, J. Kim, P.-L. Taberna, S. H. Tolbert, H. D. Abruña, P. Simon and B. Dunn, Nat. Mater., 2013, 12, 518 CrossRef CAS PubMed.
- T. Rauhala, J. Leis, T. Kallio and K. Vuorilehto, J. Power Sources, 2016, 331, 156–166 CrossRef CAS.
- I. Plitz, A. DuPasquier, F. Badway, J. Gural, N. Pereira, A. Gmitter and G. G. Amatucci, Appl. Phys. A: Mater. Sci. Process., 2005, 82, 615–626 CrossRef.
- P. Sennu, V. Aravindan, M. Ganesan, Y.-G. Lee and Y.-S. Lee, ChemSusChem, 2016, 9, 849 CrossRef CAS PubMed.
- K. Chen, J. Cao, Q. Lu, Q. Wang, M. Yao, M. Han, Z. Niu and J. Chen, Nano Res., 2017, 11, 1345–1357 CrossRef.
- L. Liu, Z. Niu and J. Chen, Chem. Soc. Rev., 2016, 45, 4340–4363 RSC.
- K. Shehzad, Y. Xu, C. Gao and X. Duan, Chem. Soc. Rev., 2016, 45, 5541–5588 RSC.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- M. Yang and Z. Zhou, Adv. Sci., 2017, 4, 1600408 CrossRef PubMed.
- J. Cao, C. Chen, Q. Zhao, N. Zhang, Q. Lu, X. Wang, Z. Niu and J. Chen, Adv. Mater., 2016, 28, 9629–9636 CrossRef CAS PubMed.
- Z. Niu, L. Liu, L. Zhang and X. Chen, Small, 2014, 10, 3434–3441 CrossRef CAS PubMed.
- L. Liu, Z. Niu, L. Zhang and X. Chen, Small, 2014, 10, 2200–2214 CrossRef CAS PubMed.
- S. R. Sivakkumar and A. G. Pandolfo, Electrochim. Acta, 2012, 65, 280–287 CrossRef CAS.
- S. Nagpure, Q. Zhang, M. A. Khan, S. Z. Islam, J. Xu, J. Strzalka, Y.-T. Cheng, B. L. Knutson and S. E. Rankin, Adv. Funct. Mater., 2018, 28, 1801849 CrossRef.
- V. Khomenko, E. Raymundo-Piñero and F. Béguin, J. Power Sources, 2008, 177, 643–651 CrossRef CAS.
- H. Park, M. Kim, F. Xu, C. Jung, S. M. Hong and C. M. Koo, J. Power Sources, 2015, 283, 68–73 CrossRef CAS.
- S. S. Zhang, J. Power Sources, 2017, 343, 322–328 CrossRef CAS.
- S. Li and X. Wang, J. Power Sources, 2015, 282, 394–400 CrossRef CAS.
- Q. Wang, X. Wang, F. Wan, K. Chen, Z. Niu and J. Chen, Small, 2018, 14, e1800280 CrossRef PubMed.
- X. Wang, F. Wan, L. Zhang, Z. Zhao, Z. Niu and J. Chen, Adv. Funct. Mater., 2018, 28, 1707247 CrossRef.
- J. H. Lee, W. H. Shin, M. H. Ryou, J. K. Jin, J. Kim and J. W. Choi, ChemSusChem, 2012, 5, 2328–2333 CrossRef CAS PubMed.
- T. Zhang, F. Zhang, L. Zhang, Y. Lu, Y. Zhang, X. Yang, Y. Ma and Y. Huang, Carbon, 2015, 92, 106–118 CrossRef CAS.
- W. Ahn, D. U. Lee, G. Li, K. Feng, X. Wang, A. Yu, G. Lui and Z. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 25297–25305 CrossRef CAS PubMed.
- Y. Sun, J. Tang, F. Qin, J. Yuan, K. Zhang, J. Li, D.-M. Zhu and L.-C. Qin, J. Mater. Chem. A, 2017, 5, 13601 RSC.
- B. Anothumakkool, N. Dupré, P. Moreau, D. Guyomard, T. Brousse and J. Gaubicher, J. Power Sources, 2018, 378, 628–635 CrossRef CAS.
- R. Tjandra, W. Liu, L. Lim and A. Yu, Carbon, 2018, 129, 152–158 CrossRef CAS.
- C. Chen, J. Cao, X. Wang, Q. Lu, M. Han, Q. Wang, H. Dai, Z. Niu, J. Chen and S. Xie, Nano Energy, 2017, 42, 187–194 CrossRef CAS.
- S. R. Sivakkumar and A. G. Pandolfo, J. Appl. Electrochem., 2013, 44, 105–113 CrossRef.
- L. Liu, Z. Niu and J. Chen, Nano Res., 2017, 10, 1524–1544 CrossRef.
- P. Luan, N. Zhang, W. Zhou, Z. Niu, Q. Zhang, L. Cai, X. Zhang, F. Yang, Q. Fan, W. Zhou, Z. Xiao, X. Gu, H. Chen, K. Li, S. Xiao, Y. Wang, H. Liu and S. Xie, Adv. Funct. Mater., 2016, 26, 8178–8184 CrossRef CAS.
- B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang and M. Cai, Energy Environ. Sci., 2016, 9, 102 RSC.
- H. Wang, Z. Li and D. Mitlin, ChemElectroChem, 2014, 1, 332–337 CrossRef.
- S. Jayaraman, A. Jain, M. Ulaganathan, E. Edison, M. P. Srinivasan, R. Balasubramanian, V. Aravindan and S. Madhavi, Chem. Eng. J., 2017, 316, 506–513 CrossRef CAS.
- Z. Yang, H. Guo, X. Li, Y. Wang, Z. Yan, D. Zhang, Z. Wang and J. Wang, J. Mater. Chem. A, 2017, 5, 15302 RSC.
- H. Kim, M.-Y. Cho, M.-H. Kim, K.-Y. Park, H. Gwon, Y. Lee, K. C. Roh and K. Kang, Adv. Energy Mater., 2013, 3, 1500–1506 CrossRef CAS.
- H. Song, J. Fu, K. Ding, C. Huang, K. Wu, X. Zhang, B. Gao, K. Huo, X. Peng and P. K. Chu, J. Power Sources, 2016, 328, 599–606 CrossRef CAS.
- C. Zhang, M. Beidaghi, M. Naguib, M. R. Lukatskaya, M.-Q. Zhao, B. Dyatkin, K. M. Cook, S. J. Kim, B. Eng, X. Xiao, D. Long, W. Qiao, B. Dunn and Y. Gogotsi, Chem. Mater., 2016, 28, 3937–3943 CrossRef CAS.
- B. Deng, T. Lei, W. Zhu, L. Xiao and J. Liu, Adv. Funct. Mater., 2018, 28, 1704330 CrossRef.
- E. Kim, H. Kim, B. J. Park, Y. H. Han, J. H. Park, J. Cho, S. S. Lee and J. G. Son, Small, 2018, 14, e1704209 CrossRef PubMed.
- L. Zhao, Y. S. Hu, H. Li, Z. Wang and L. Chen, Adv. Mater., 2011, 23, 1385–1388 CrossRef CAS PubMed.
- K. Naoi, S. Ishimoto, Y. Isobe and S. Aoyagi, J. Power Sources, 2010, 195, 6250–6254 CrossRef CAS.
- H. S. Choi, J. H. Im, T. Kim, J. H. Park and C. R. Park, J. Mater. Chem., 2012, 22, 16986 RSC.
- B.-G. Lee and J.-R. Yoon, J. Electr. Eng. Technol., 2012, 7, 207–211 CrossRef.
- S. Zheng, J. Ma, Z.-S. Wu, F. Zhou, Y.-B. He, F. Kang, H.-M. Cheng and X. Bao, Energy Environ. Sci., 2018, 11, 2001 RSC.
- J. Kang, S.-H. Wei, K. Zhu and Y.-H. Kim, J. Phys. Chem. C, 2011, 115, 4909–4915 CrossRef CAS.
- L. F. Que, F. D. Yu, Z. B. Wang and D. M. Gu, Small, 2018, 14, e1704508 CrossRef PubMed.
- K. Leng, F. Zhang, L. Zhang, T. Zhang, Y. Wu, Y. Lu, Y. Huang and Y. Chen, Nano Res., 2013, 6, 581–592 CrossRef CAS.
- S. Dsoke, B. Fuchs, E. Gucciardi and M. Wohlfahrt-Mehrens, J. Power Sources, 2015, 282, 385–393 CrossRef CAS.
- N. Xu, X. Sun, X. Zhang, K. Wang and Y. Ma, RSC Adv., 2015, 5, 94361 RSC.
- L. Ye, Q. Liang, Y. Lei, X. Yu, C. Han, W. Shen, Z.-H. Huang, F. Kang and Q.-H. Yang, J. Power Sources, 2015, 282, 174–178 CrossRef CAS.
- H. Zhao, X. Ma, J. Bai, Z. Yang, G. Sun, Z. Zhang, X. Pan, W. Lan, J. Y. Zhou and E. Xie, Nanoscale, 2017, 9, 8192–8199 RSC.
- K. Naoi, T. Kurita, M. Abe, T. Furuhashi, Y. Abe, K. Okazaki, J. Miyamoto, E. Iwama, S. Aoyagi, W. Naoi and P. Simon, Adv. Mater., 2016, 28, 6751–6757 CrossRef CAS PubMed.
- C. Yang, J. L. Lan, W. X. Liu, Y. Liu, Y. H. Yu and X. P. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 18710–18719 CrossRef CAS PubMed.
- S. Dong, H. Li, J. Wang, X. Zhang and X. Ji, Nano Res., 2017, 10, 4448–4456 CrossRef CAS.
- Y. Qian, X. Cai, C. Zhang, H. Jiang, L. Zhou, B. Li and L. Lai, Electrochim. Acta, 2017, 258, 1311–1319 CrossRef CAS.
- Y. Xie and R. Gao, J. Alloys Compd., 2017, 725, 1–13 CrossRef CAS.
- G. Tang, L. Cao, P. Xiao, Y. Zhang and H. Liu, J. Power Sources, 2017, 355, 1–7 CrossRef CAS.
- F. Wang, C. Wang, Y. Zhao, Z. Liu, Z. Chang, L. Fu, Y. Zhu, Y. Wu and D. Zhao, Small, 2016, 12, 6207–6213 CrossRef CAS PubMed.
- X. Wang, Y. Li, T. Jin, J. Meng, L. Jiao, M. Zhu and J. Chen, Nano Lett., 2017, 17, 7989–7994 CrossRef CAS PubMed.
- C. Chen, J. Cao, Q. Lu, X. Wang, L. Song, Z. Niu and J. Chen, Adv. Funct. Mater., 2017, 27, 1604639 CrossRef.
- C.-L. Hsieh, D.-S. Tsai, W.-W. Chiang and Y.-H. Liu, Electrochim. Acta, 2016, 209, 332–340 CrossRef CAS.
- P. Wang, G. Zhang, H. Jiao, W. Chen, L. Liu, X. Wang, Q. Chen, X. Deng and X. Zheng, Appl. Surf. Sci., 2019, 466, 724–729 CrossRef CAS.
- X. Zhao, C. Johnston and P. S. Grant, J. Mater. Chem., 2009, 19, 8755 RSC.
- R. V. Salvatierra, D. Zakhidov, J. Sha, N. D. Kim, S. K. Lee, A. O. Raji, N. Zhao and J. M. Tour, ACS Nano, 2017, 11, 2724–2733 CrossRef CAS PubMed.
- Z. Liu, Z. Zhao, Y. Wang, S. Dou, D. Yan, D. Liu, Z. Xia and S. Wang, Adv. Mater., 2017, 29, 1606207 CrossRef PubMed.
- M. Li, Y. Yang, E. S. Guang Choo, X. Tang, K. Sun, Y. Chen and J. Xue, J. Power Sources, 2018, 394, 131–139 CrossRef CAS.
- L. P. Wang, L. Yu, R. Satish, J. Zhu, Q. Yan, M. Srinivasan and Z. Xu, RSC Adv., 2014, 4, 37389 RSC.
- P. Arunkumar, A. G. Ashish, B. Babu, S. Sarang, A. Suresh, C. H. Sharma, M. Thalakulam and M. M. Shaijumon, RSC Adv., 2015, 5, 59997 RSC.
- R. Wang, J. Lang, P. Zhang, Z. Lin and X. Yan, Adv. Funct. Mater., 2015, 25, 2270–2278 CrossRef CAS.
- M. Liu, L. Zhang, P. Han, X. Han, H. Du, X. Yue, Z. Zhang, H. Zhang and G. Cui, Part. Part. Syst. Charact., 2015, 32, 1006–1011 CrossRef CAS.
- H. Wang, C. Guan, X. Wang and H. J. Fan, Small, 2015, 11, 1470–1477 CrossRef CAS PubMed.
- Z. Chen, Y. Yuan, H. Zhou, X. Wang, Z. Gan, F. Wang and Y. Lu, Adv. Mater., 2014, 26, 339–345 CrossRef CAS PubMed.
- B.-G. Lee, S.-H. Lee, H.-J. Ahn and J.-R. Yoon, J. Alloys Compd., 2018, 748, 882–888 CrossRef CAS.
- G. Wang, C. Lu, X. Zhang, B. Wan, H. Liu, M. Xia, H. Gou, G. Xin, J. Lian and Y. Zhang, Nano Energy, 2017, 36, 46–57 CrossRef CAS.
- L. Gao, D. Huang, Y. Shen and M. Wang, J. Mater. Chem. A, 2015, 3, 23570 RSC.
- H. Kim, K.-Y. Park, M.-Y. Cho, M.-H. Kim, J. Hong, S.-K. Jung, K. C. Roh and K. Kang, ChemElectroChem, 2014, 1, 125–130 CrossRef.
- S. Deng, J. Li, S. Sun, H. Wang, J. Liu and H. Yan, Electrochim. Acta, 2014, 146, 37–43 CrossRef CAS.
- R. Gokhale, V. Aravindan, P. Yadav, S. Jain, D. Phase, S. Madhavi and S. Ogale, Carbon, 2014, 80, 462–471 CrossRef CAS.
- T. Liang, H. Wang, D. Xu, K. Liao, R. Wang, B. He, Y. Gong and C. Yan, Nanoscale, 2018, 10, 17814–17823 RSC.
- C. Jiang, J. Zhao, H. Wu, Z. Zou and R. Huang, J. Power Sources, 2018, 401, 135–141 CrossRef CAS.
- J. Wang, H. Li, L. Shen, S. Dong and X. Zhang, RSC Adv., 2016, 6, 71338 RSC.
- S. Liu, X. Xia, Y. Zhong, S. Deng, Z. Yao, L. Zhang, X.-B. Cheng, X. Wang, Q. Zhang and J. Tu, Adv. Energy Mater., 2018, 8, 1702322 CrossRef.
- H. Wang, Y. Zhang, H. Ang, Y. Zhang, H. T. Tan, Y. Zhang, Y. Guo, J. B. Franklin, X. L. Wu, M. Srinivasan, H. J. Fan and Q. Yan, Adv. Funct. Mater., 2016, 26, 3082–3093 CrossRef CAS.
- J. Luo, W. Zhang, H. Yuan, C. Jin, L. Zhang, H. Huang, C. Liang, Y. Xia, J. Zhang, Y. Gan and X. Tao, ACS Nano, 2017, 11, 2459–2469 CrossRef CAS PubMed.
- L. Wang, J. Sun, R. Song, S. Yang and H. Song, Adv. Energy Mater., 2016, 6, 1502067 CrossRef.
- R. Satish, V. Aravindan, W. C. Ling and S. Madhavi, J. Power Sources, 2015, 281, 310–317 CrossRef CAS.
- X. Wang and G. Shen, Nano Energy, 2015, 15, 104–115 CrossRef.
- J. H. Won, H. M. Jeong and J. K. Kang, Adv. Energy Mater., 2017, 7, 1601355 CrossRef.
- C. H. Lai, D. Ashby, M. Moz, Y. Gogotsi, L. Pilon and B. Dunn, Langmuir, 2017, 33, 9407–9415 CrossRef CAS PubMed.
- E. Lim, C. Jo, H. Kim, M.-H. Kim, Y. Mun, J. Chun, Y. Ye, J. Hwang, K.-S. Ha, K. K. K. C. Roh, S. Yoon and J. Lee, ACS Nano, 2015, 9, 7497 CrossRef CAS PubMed.
- E. Lim, H. Kim, C. Jo, J. Chun, K. Ku, S. Kim, H. I. Lee, I.-S. Nam, S. Yoon, K. Kang and J. Lee, ACS Nano, 2014, 8, 8968–8978 CrossRef CAS PubMed.
- L. Kong, C. Zhang, J. Wang, W. Qiao, L. Ling and D. Long, ACS Nano, 2015, 9, 11200–11208 CrossRef CAS PubMed.
- X. Wang, G. Li, Z. Chen, V. Augustyn, X. Ma, G. Wang, B. Dunn and Y. Lu, Adv. Energy Mater., 2011, 1, 1089–1093 CrossRef CAS.
- X. Yu, J. Deng, C. Zhan, R. Lv, Z.-H. Huang and F. Kang, Electrochim. Acta, 2017, 228, 76–81 CrossRef CAS.
- M. Li, F. Pan, E. S. G. Choo, Y. Lv, Y. Chen and J. Xue, ACS Appl. Mater. Interfaces, 2016, 8, 6972–6981 CrossRef CAS PubMed.
- A. Brandt and A. Balducci, Electrochim. Acta, 2013, 108, 219–225 CrossRef CAS.
- F. Zhang, T. Zhang, X. Yang, L. Zhang, K. Leng, Y. Huang and Y. Chen, Energy Environ. Sci., 2013, 6, 1623 RSC.
- P. Wang, R. Wang, J. Lang, X. Zhang, Z. Chen and X. Yan, J. Mater. Chem. A, 2016, 4, 9760 RSC.
- Z.-K. Chen, J.-W. Lang, L.-Y. Liu and L.-B. Kong, RSC Adv., 2017, 7, 19967 RSC.
- M. Secchiaroli, R. Marassi, M. Wohlfahrt-Mehrens and S. Dsoke, Electrochim. Acta, 2016, 219, 425–434 CrossRef CAS.
- V. Aravindan, W. Chuiling, M. V. Reddy, G. V. Rao, B. V. Chowdari and S. Madhavi, Phys. Chem. Chem. Phys., 2012, 14, 5808–5814 RSC.
- X. Jiao, Q. Hao, X. Xia, D. Yao, Y. Ouyang and W. Lei, J. Power Sources, 2018, 403, 66–75 CrossRef CAS.
- V. Aravindan, M. V. Reddy, S. Madhavi, S. G. Mhaisalkar, G. V. Subba Rao and B. V. R. Chowdari, J. Power Sources, 2011, 196, 8850–8854 CrossRef CAS.
- C.-J. Peng, D.-S. Tsai, C.-H. Chang and H.-Y. Wei, J. Power Sources, 2015, 274, 15–21 CrossRef CAS.
- C.-J. Peng, D.-S. Tsai, C.-H. Chang and M.-V. Le, J. Alloys Compd., 2015, 627, 186–191 CrossRef CAS.
- T. Shen, Z. Yao, X. Xia, X. Wang, C. Gu and J. Tu, Adv. Eng. Mater., 2018, 20, 1700591 CrossRef.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- B. Wang, X. Li, B. Luo, L. Hao, M. Zhou, X. Zhang, Z. Fan and L. Zhi, Adv. Mater., 2015, 27, 1526–1532 CrossRef CAS PubMed.
- K. Feng, M. Li, W. Liu, A. G. Kashkooli, X. Xiao, M. Cai and Z. Chen, Small, 2018, 14, 1702737 CrossRef PubMed.
- R. Yi, S. Chen, J. Song, M. L. Gordin, A. Manivannan and D. Wang, Adv. Funct. Mater., 2014, 24, 7433–7439 CrossRef CAS.
- B. Li, S. Li, Y. Jin, J. Zai, M. Chen, A. Nazakat, P. Zhan, Y. Huang and X. Qian, J. Mater. Chem. A, 2018, 6, 21098–21103 RSC.
- G. P. Pandey, S. A. Klankowski, T. Liu, J. Wu and J. Li, J. Power Sources, 2017, 342, 1006–1016 CrossRef CAS.
- L. Zhou, K. Zhang, Z. Hu, Z. Tao, L. Mai, Y.-M. Kang, S.-L. Chou and J. Chen, Adv. Energy Mater., 2018, 8, 1701415 CrossRef.
- Y. Xu, Q. Liu, Y. Zhu, Y. Liu, A. Langrock, M. R. Zachariah and C. Wang, Nano Lett., 2013, 13, 470–474 CrossRef CAS PubMed.
- G. Derrien, J. Hassoun, S. Panero and B. Scrosati, Adv. Mater., 2007, 19, 2336–2340 CrossRef CAS.
- F. Sun, J. Gao, Y. Zhu, X. Pi, L. Wang, X. Liu and Y. Qin, Sci. Rep., 2017, 7, 40990 CrossRef CAS PubMed.
- C. Wang, Y. Han, S. Li, T. Chen, J. Yu and Z. Lu, ACS Appl. Mater. Interfaces, 2018, 10, 12750–12758 CrossRef CAS PubMed.
- C. S. Yoon, S. J. Kim, U.-H. Kim, K.-J. Park, H.-H. Ryu, H.-S. Kim and Y.-K. Sun, Adv. Funct. Mater., 2018, 28, 1802090 CrossRef.
- Y. Yang, X. Qu, L. Zhang, M. Gao, Y. Liu and H. Pan, ACS Appl. Mater. Interfaces, 2018, 10, 20591–20598 CrossRef CAS PubMed.
- A. Varzi, C. Ramirez-Castro, A. Balducci and S. Passerini, J. Power Sources, 2015, 273, 1016–1022 CrossRef CAS.
- M. K. Singh and S. A. Hashmi, Ionics, 2017, 23, 2931–2942 CrossRef CAS.
- J. Feng, N. A. Chernova, F. Omenya, L. Tong, A. C. Rastogi and M. Stanley Whittingham, J. Solid State Electrochem., 2017, 22, 1063–1078 CrossRef.
- K. Kisu, E. Iwama, W. Naoi, P. Simon and K. Naoi, Electrochem. Commun., 2016, 72, 10–14 CrossRef CAS.
- K. Naoi, K. Kisu, E. Iwama, S. Nakashima, Y. Sakai, Y. Orikasa, P. Leone, N. Dupré, T. Brousse, P. Rozier, W. Naoi and P. Simon, Energy Environ. Sci., 2016, 9, 2143–2151 RSC.
- X.-L. Wu, L.-Y. Jiang, F.-F. Cao, Y.-G. Guo and L.-J. Wan, Adv. Mater., 2009, 21, 2710–2714 CrossRef CAS.
- Z. Jian, Y. S. Hu, X. Ji and W. Chen, Adv. Mater., 2017, 29, 1601925 CrossRef PubMed.
- Y. Liu, B. Yang, X. Dong, Y. Wang and Y. Xia, Angew. Chem., Int. Ed., 2017, 56, 16606–16610 CrossRef CAS PubMed.
- J. Jiang, G. Tan, S. Peng, D. Qian, J. Liu, D. Luo and Y. Liu, Electrochim. Acta, 2013, 107, 59–65 CrossRef CAS.
- N.-W. Li, X. Du, J.-L. Shi, X. Zhang, W. Fan, J. Wang, S. Zhao, Y. Liu, W. Xu, M. Li, Y.-G. Guo and C. Li, Electrochim. Acta, 2018, 281, 459–465 CrossRef CAS.
- V. Aravindan, W. Chuiling and S. Madhavi, J. Mater. Chem., 2012, 22, 16026 RSC.
- S. Zhang, C. Li, X. Zhang, X. Sun, K. Wang and Y. Ma, ACS Appl. Mater. Interfaces, 2017, 9, 17136–17144 CrossRef CAS PubMed.
- H. Du, H. Yang, C. Huang, J. He, H. Liu and Y. Li, Nano Energy, 2016, 22, 615–622 CrossRef CAS.
- N. Arun, A. Jain, V. Aravindan, S. Jayaraman, W. Chui Ling, M. P. Srinivasan and S. Madhavi, Nano Energy, 2015, 12, 69–75 CrossRef CAS.
- D. P. Dubal and P. Gomez-Romero, Mater Today Energy., 2018, 8, 109–117 CrossRef.
- X.-Y. Shan, Y. Wang, D.-W. Wang, F. Li and H.-M. Cheng, Adv. Energy Mater., 2016, 6, 1502064 CrossRef.
- X. Yu, C. Zhan, R. Lv, Y. Bai, Y. Lin, Z.-H. Huang, W. Shen, X. Qiu and F. Kang, Nano Energy, 2015, 15, 43–53 CrossRef CAS.
- X. Han, P. Han, J. Yao, S. Zhang, X. Cao, J. Xiong, J. Zhang and G. Cui, Electrochim. Acta, 2016, 196, 603–610 CrossRef CAS.
- J. J. Ren, L. W. Su, X. Qin, M. Yang, J. P. Wei, Z. Zhou and P. W. Shen, J. Power Sources, 2014, 264, 108–113 CrossRef CAS.
- J. H. Lee, W. H. Shin, S. Y. Lim, B. G. Kim and J. W. Choi, Mater. Renewable Sustainable Energy, 2014, 3, 22–29 CrossRef.
- M. Schroeder, M. Winter, S. Passerini and A. Balducci, J. Power Sources, 2013, 238, 388–394 CrossRef CAS.
- R. Shi, C. Han, H. Li, L. Xu, T. Zhang, J. Li, Z. Lin, C.-P. Wong, F. Kang and B. Li, J. Mater. Chem. A, 2018, 6, 17057–17066 RSC.
- A. Jain, V. Aravindan, S. Jayaraman, P. S. Kumar, R. Balasubramanian, S. Ramakrishna, S. Madhavi and M. P. Srinivasan, Sci. Rep., 2013, 3, 3002 CrossRef PubMed.
- V. Aravindan, J. Sundaramurthy, A. Jain, P. S. Kumar, W. C. Ling, S. Ramakrishna, M. P. Srinivasan and S. Madhavi, ChemSusChem, 2014, 7, 1858–1863 CrossRef CAS PubMed.
- L. Sun, W. Liu, Y. Cui, Y. Zhang, H. Wang, S. Liu and B. Shan, Green Chem., 2018, 20, 3954–3962 RSC.
- A. Banerjee, K. K. Upadhyay, D. Puthusseri, V. Aravindan, S. Madhavi and S. Ogale, Nanoscale, 2014, 6, 4387–4394 RSC.
- J. Xu, Y. Li, L. Wang, Q. Cai, Q. Li, B. Gao, X. Zhang, K. Huo and P. K. Chu, Nanoscale, 2016, 8, 16761–16768 RSC.
- V. Aravindan, D. Mhamane, W. C. Ling, S. Ogale and S. Madhavi, ChemSusChem, 2013, 6, 2240–2244 CrossRef CAS PubMed.
- X. Ma, L. Zhao, X. Song, Z. Yu, L. Zhao, Y. Yu, Z. Xiao, G. Ning and J. Gao, Carbon, 2018, 140, 314–323 CrossRef CAS.
- D. Mhamane, V. Aravindan, M.-S. Kim, H.-K. Kim, K. C. Roh, D. Ruan, S. H. Lee, M. Srinivasan and K.-B. Kim, J. Mater. Chem. A, 2016, 4, 5578 RSC.
- R. Shi, C. Han, X. Xu, X. Qin, L. Xu, H. Li, J. Li, C. P. Wong and B. Li, Chemistry, 2018, 24, 10460–10467 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2019 |