Fluorene-fused ladder-type non-fullerene small molecule acceptors for high-performance polymer solar cells†
Ruijie
Ming
a,
Miao
Zhang
b,
Wei
Gao
a,
Weimin
Ning
a,
Zhenghui
Luo
a,
Cheng
Zhong
a,
Fujun
Zhang
 *b and
Chuluo
Yang
*b and
Chuluo
Yang
 *a
*a
aHubei Key Lab on Organic and Polymeric Optoelectronic Materials, Department of Chemistry, Wuhan University, Wuhan, 430072, People's Republic of China. E-mail: clyang@whu.edu.cn
bKey Laboratory of Luminescence and Optical Information, Ministry of Education, Beijing Jiaotong University, Beijing, 100044, People's Republic of China. E-mail: fjzhang@bjtu.edu.cn
First published on 5th March 2019
Abstract
In this work, we designed and synthesized two new small molecule acceptors (SMAs), namely FTTCN and FTTCN-M, structured with a coplanar central core based on a fluorene-fused ladder-type donor and electron-withdrawing end-capping groups (CPTCN and CPTCN-M). Compared to FTTCN-M, FTTCN exhibits slightly redshifted absorption, higher electron mobility and a more favorable morphology of the blend film. FTTCN-based polymer solar cells (PSCs) paired with p-type polymer PBDB-T achieved a power conversion efficiency (PCE) of 10.56%, with an open-circuit voltage (VOC) of 0.90 V, a short-circuit current density (JSC) of 15.89 mA cm−2 and a fill factor (FF) of 73.82%, which is slightly higher than that of FTTCN-M-based devices (a PCE of 10.08% alone with a VOC of 0.93 V, a JSC of 15.19 mA cm−2 and a FF of 71.37%). To the best of our knowledge, the FF of 73.82% is the highest value for fluorene-based PSCs reported to date. The higher JSC and FF of the FTTCN-based device could be attributed to the lower charge recombination, more balanced carrier mobility, and more favorable surface morphology of the PBDB-T:FTTCN blend film than those of the PBDB-T:FTTCN-M blend film. This work revealed that a fluorene-fused ladder-type donor exhibits great potential in constructing high-performance non-fullerene SMAs and optimizing end-capping groups.
Introduction
Bulk heterojunction organic photovoltaic cells (OPVs) have attracted increasing attention due to their advantages of large area, light weight and flexibility.1–7 Great efforts have been made towards developing high-performance polymer solar cells (PSCs) over the last two decades. The power conversion efficiencies (PCEs) of PSCs with fullerene derivatives as electron acceptors (i.e. [6,6]-phenyl-C61/C71-butyric acid methyl ester, PC61BM and PC71BM) have broken through 11%.8–14 However, fullerene-based acceptors also have several inherent defects, such as excessive aggregation at high temperature, narrow and weak absorption in the visible region, and limited energy level variability, which hinder the continuous advances of fullerene-based PSCs. As a new dawn, non-fullerene acceptors (NFAs) with merits of broad absorption ranges, appropriate energy levels and suitable crystallization properties have emerged as a class of promising SMAs, which can be developed through multiple molecular engineering and chemical modifications. Perylene diimide (PDI) and naphthalene diimide (NDI) with twisted three-dimensional structures have been widely used as NFAs with good photovoltaic performances and show PCEs over 10% in PSC devices.15–20 Among those reported, NFAs with acceptor–donor–acceptor (A–D–A)-type structures have been demonstrated to be the most potential SMAs to date. Since Zhan et al. first reported a fused-ring electron acceptor (FREA), 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene (ITIC),21 great advances have been made, and PSCs based on ITIC and its analogues as electron acceptors have achieved PCEs of up to 14% in single-junction devices,22–35 and show great progress compared with fullerene-based PSCs.Generally, FREAs consist of a ladder-type donor, end-capping groups and side chains. The electron-donating core affords a planar conjugated center to facilitate molecule packing and can extend light absorption through versatile modification. In addition, the side chains linked to the central core by sp3 hybrid carbon atoms can not only improve solubility, but also restrain severe aggregation. Furthermore, the electron-withdrawing end-capping groups can effectively tune the energy levels and induce intense intramolecular charge transfer (ICT).36–38 Taking them into consideration, designing π-conjugated aromatic cores, terminal groups, and side chain substituents is the main modification strategy for ideal molecules to achieve high-performance PSCs. It is worth noting that the modulation of π-conjugated aromatic cores is regarded as the most universal method for developing high-performance PSCs.39–42
Fluorenedicyclopentathiophene (FT)-based SMAs have been widely investigated in PSCs, such as fused-ring engineering for broadening absorption spectra and side chain engineering for adjusting crystallinity and intermolecular π–π stacking.43,44 Considering the weak electron-donating effect of fluorene, FT-based PSCs usually possess high VOC due to the high-lying lowest unoccupied molecular orbital (LUMO) energy levels of FT-based SMAs, which would be a viable strategy to design high-performance PSCs by increasing short-circuit current density (JSC) without reducing the high VOC of FT-based PSCs. Fluorenedicyclopentathieno[3,2-b]thiophene (FTT), as a weak electron-donating group, bears a more extended coplanar and conjugated structure than that of FT, where the two thieno[3,2-b]thiophene units are covalently bonded to the central fluorene core. The coplanar and extended conjugated structure, equipped with six side chains, can ensure sufficient solubility for the solution process.
In this study, we designed and synthesized two SMAs, namely FTTCN and FTTCN-M, structured with a coplanar central core FTT backbone and 2-(6-oxo-5,6-dihydro-4H-cyclopenta[c]thiophen-4-ylidene)malononitrile (CPTCN)- and 2-(1-methyl-6-oxo-5,6-dihydro-4H-cyclopenta[c]thiophen-4-ylidene)malononitrile (CPTCN-M)-end groups.45 Poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-d′-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))] (PBDB-T) was chosen as the polymer donor to investigate the photovoltaic performance of FTTCN- and FTTCN-M-based PSCs. The optimized PBDB-T:FTTCN-based device exhibited a high PCE of 10.56% with a VOC of 0.90 V, a JSC of 15.89 mA cm−2 and a FF of 73.82%, which is slightly higher than that of the PSC based on PBDB-T:FTTCN-M (a PCE of 10.08%). To the best of our knowledge, the FF of 73.82% is the highest value for fluorene-based PSCs reported to date. Furthermore, in order to fully understand the two novel fluorene-fused ladder-type SMAs, we systematically studied the optical and electrochemical properties, photovoltaic performance, exciton dissociation and charge collection behavior, charge mobility, and film morphologies of the two SMA based PSC devices. This work revealed that the fluorene-fused ladder-type donor is a kind of potential building block for high-performance non-fullerene SMAs and optimizing end groups can further enhance the PCE of PSCs.
Results and discussion
Synthesis and characterization
The chemical structures and the synthetic routes of FTTCN and FTTCN-M are depicted in Scheme 1. Compound 1 was synthesized by Suzuki cross-coupling under Pd(PPh3)4 catalysis. Then the key intermediate with two ester groups was subjected to acid-mediated intramolecular Friedel–Crafts reactions to form compound 2. Double formyl groups of compound 3 were introduced by the Vilsmeier–Haack reaction in the presence of N,N-dimethylformamide (DMF) and phosphorus oxychloride (POCl3). High-performance Knoevenagel condensation reactions between compound 3 and CPTCN (and CPTCN-M) were conducted to afford target products FTTCN (and FTTCN-M), respectively. The target products showed good solubility in common organic solvents, such as chloroform (CF), chlorobenzene (CB) and o-dichlorobenzene (o-DCB). The chemical structures of the intermediates and target products were fully characterized by 1H NMR, 13C NMR, and HRMS (ESI)/MALDI-TOF-MS. Both FTTCN and FTTCN-M showed good thermal stability with decomposition temperatures (5% weight loss) over 340 °C (340 °C for FTTCN and 345 °C for FTTCN-M) under a nitrogen atmosphere (Fig. S1, ESI†).Optical properties
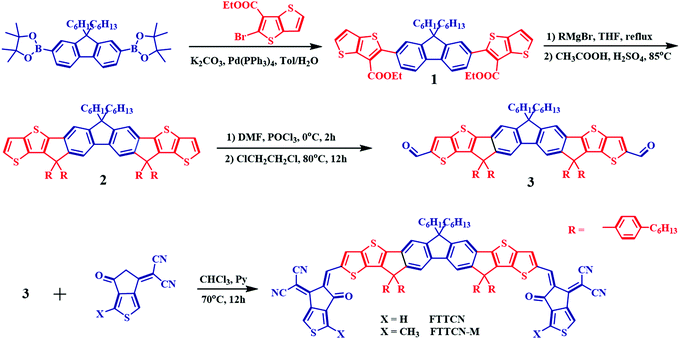
The UV-vis absorption measurements of the two SMAs were performed in CF solution and as neat films (Fig. 1a and b) and the relevant data are listed in Table 1. In the dilute CF solution, FTTCN and FTTCN-M displayed similar and strong absorption spectra with a wide absorption range from 500 to 750 nm. Compared to FTTCN, FTTCN-M showed slightly blue-shifted absorption, which could be ascribed to the weak ICT caused by the tiny electron donating effect of methyl. Meanwhile, FTTCN-M displayed a maximum molar extinction coefficient (εmax) of 1.8 × 105 M−1 cm−1 at 681 nm, while the εmax value of FTTCN is 1.4 × 105 M−1 cm−1 at 687 nm (Fig. S2, ESI†). The corresponding absorption spectra of the two SMAs as films were largely red-shifted with respect to their solution absorption, indicating intense intermolecular π–π stacking. In addition, a common wide-bandgap polymer, PBDB-T, was selected as a donor to pair with the two acceptors due to the complementary absorption for PSC device fabrication.| Acceptor | λ onset (nm) | ε max (M−1 cm−1) | λ max (nm) | λ onset (nm) | λ max (nm) |
E
optg
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (eV)
(eV) |
HOMOd (eV) | LUMOd (eV) |
E
cvg
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (eV)
(eV) |
|---|---|---|---|---|---|---|---|---|---|
| a In CF solution. b In thin films drop-cast from chloroform solution. c Estimated from the empirical formula: Eoptg = 1240/λonset. d Cyclic voltammetry (CV) method. e E CVg = ELUMO − EHOMO. | |||||||||
| FTTCN | 743 | 1.4 × 105 | 687 | 794 | 706 | 1.56 | −5.54 | −3.95 | 1.59 |
| FTTCN-M | 732 | 1.8 × 105 | 681 | 791 | 703 | 1.57 | −5.53 | −3.91 | 1.62 |
Electrochemical properties and theoretical calculations
The HOMO and LUMO energy levels of FTTCN and FTTCN-M were investigated by the cyclic voltammetry (CV) method (Fig. S2, ESI†). The cycle volt–ampere characteristic curves showed irreversible oxidation and reduction waves, and the oxidation/reduction onset potentials for FTTCN and FTTCN-M versus Ag/AgCl were found to be 1.34 eV/−0.25 eV and 1.33 eV/−0.29 eV, respectively. The assumed vacuum energy level and the measured relative energy level of Fc/Fc+ were −4.8 eV and 0.60 eV, respectively. As a result, the corresponding HOMO and LUMO levels of FTTCN and FTTCN-M were calculated to be −5.54 eV/−3.95 eV and −5.53 eV/−3.91 eV, respectively, suggesting that the methylated end-capping group CPTCN-M could upshift both the HOMO and LUMO energy levels (Fig. 1c).To fully understand the newly-developed fluorene-fused electron-donating core effect on the molecular geometry and electronic properties of the two SMAs, density functional theory (DFT) simulations at the B3LYP/6-31G(d) level were performed where the side chain groups were simplified to be methyl. The DFT calculations manifested that FTTCN and FTTCN-M possess almost the same HOMO and LUMO distributions and coplanar molecular backbones owing to their almost identical backbones and small difference in the end groups. The DFT calculations showed that the HOMO/LUMO energy levels were −5.40 eV/−3.31 eV for FTTCN and −5.37 eV/−3.27 eV for FTTCN-M (Fig. S3, ESI†). Meanwhile, it is obvious that the HOMOs were distributed almost entirely over the electron-rich fused-ring cores, whereas the LUMOs were delocalized along the whole molecular backbones, which indicates a strong ICT effect and is in favor of hole and electron transport within the molecules.
Photovoltaic performance
To gain deep insights into the photovoltaic performance of FTT-based SMAs, PSCs based on the two SMAs were fabricated with a traditional device structure of ITO/PEDOT:PSS/active layer/PDIN/Ag, where ITO (indium tin oxide) was the anode; PEDOT:PSS (poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)) and PDIN (N,N′-bis-(propylenedimethylamine)-3,4,9,10-perylenediimide) served as hole and electron transport layers (HTL and LTL), respectively; high-work-function metal of Ag (silver) was used as the cathode and-the wide-bandgap 2D-conjugated polymer PBDB-T was selected as the donor considering their complementary spectra and well-matched energy levels with FTTCN and FTTCN-M (Fig. 2a). The parameters of the best-performing devices are summarized in Table 2, and the corresponding current density–voltage (J–V) characteristics and external quantum efficiency (EQE) curves are shown in Fig. 2c and d. More detailed device parameters with parallel fabrication are provided in Fig. S4 and Table S1 (ESI†).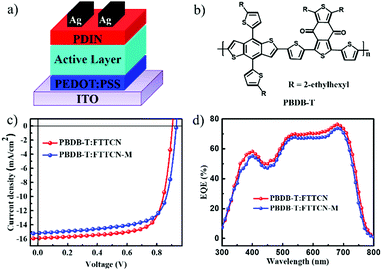 | ||
| Fig. 2 (a) Device structure of FTTCN- and FTTCN-M-based PSCs; (b) molecule structure of PBDB-T; (c) J–V curves of FTTCN- and FTTCN-M-based PSCs and (d) the corresponding EQE spectra. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) and PBDB-T:FTTCN-M (1
1 w/w) and PBDB-T:FTTCN-M (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) under AM1.5G illumination at 100 mW cm−2
1 w/w) under AM1.5G illumination at 100 mW cm−2
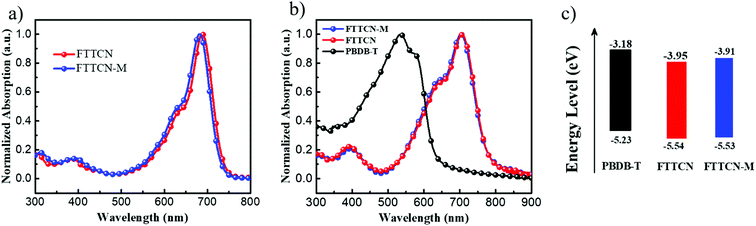
The best PCEs for the FTTCN- and FTTCN-M-based devices were found to be 10.56% and 10.08%, respectively. As shown in Fig. 2c, on the one hand, compared to the PBDB-T:FTTCN-based device, the PBDB-T:FTTCN-M-based device possessed higher VOC, which should be attributed to its higher LUMO level of FTTCN-M; on the other hand, the PBDB-T:FTTCN-M-based device showed lower JSC, which could be caused by the slightly blue-shifted absorption spectrum. As a result, the FTTCN-based PSCs exhibited a VOC of 0.90 V, a JSC of 15.89 mA cm−2 and a FF of 73.82% with a higher PCE of 10.56% than those of the FTTCN-M-based PSCs (a PCE of 10.08% with a VOC of 0.93 V, a JSC of 15.19 mA cm−2 and a FF of 71.37%). To the best of our knowledge, the FF of 73.82% is the highest value for fluorene-based PSCs reported to date (Table S4, ESI†). External quantum efficiency (EQE) measurements were also investigated to confirm the improvement of JSC, as shown in Fig. 2d. Both devices showed a broad and strong spectral response from 300 to 800 nm; a maximum EQE value of 76% at 681 nm was observed for the FTTCN devices, which was slightly higher than that of the FTTCN-M-based devices (73% at 681 nm). The JSC values calculated from the EQE curves (Jcal.) were 15.59 mA cm−2 and 14.71 mA cm−2 for the FTTCN- and FTTCN-M-based devices, respectively, which commendably corresponded to the results obtained from the J–V measurements within a 4% mismatch.
Recombination dynamics
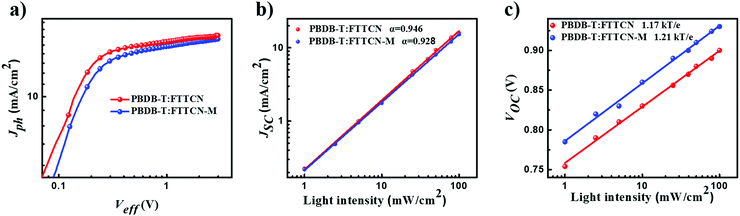
To figure out the exciton dissociation and charge collection properties, we measured the dependence of the photocurrent (Jph) on the effective voltage (Veff) (Fig. 3a). The Jph is defined as JL – JD, where JL and JD are the current densities under illumination and in the dark, and Veff is defined as V0 – Va, where V0 is the voltage at which JL = JD, and Va is the applied voltage. Jph reached saturation (Jsat) in the high reverse voltage region (Veff > 2 V), indicating that the excitons could be dissociated effectively. The exciton dissociation efficiency (ηdiss) and charge collection efficiency (ηcoll) under the short circuit and maximum power output conditions were determined (Table S2, ESI†). The ηdiss and ηcoll values were calculated to be 93.9% and 84.5% for FTTCN-based PSCs, and 92.8% and 80.2% for FTTCN-M-based PSCs, respectively, indicating improved exciton dissociation and charge collection efficiency for PBDB-T:FTTCN-based devices and thus reinforced JSC and FF.To further explore the charge recombination process in the PSC devices, JSC and VOC as functions of light intensity (Plight) were examined based on two optimized devices. The correlation between JSC and Plight was expressed as a power-law equation JSC ∝ Pαlight, where the exponential factor α indicates the extent of bimolecular recombination.46–48 The α factor should be 1 if there is no bimolecular recombination and it is smaller than 1 for the devices with bimolecular charge recombination. As shown in Fig. 3b, it can be seen that the α values were 0.946 and 0.928 for FTTCN- and FTTCN-M -based PSCs, respectively, which indicated smaller bimolecular recombination of PBDB-T:FTTCN devices than that of PBDB-T:FTTCN-M devices. The relationship between VOC and Plight can be expressed as: VOC ∝ nkT/e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Plight (Fig. 3c), where k, T, and e are the Boltzmann constant, the temperature in Kelvin, and the elementary charge, respectively. It is well known that n is close to 1, implying that bimolecular recombination is the dominant mechanism; when n is close to 2, the additional mechanism of monomolecular recombination or trap-assisted recombination is involved. The dependences of VOC on Plight for the PBDB-T:FTTCN- and PBDB-T:FTTCN-M-based PSCs exhibited low slopes of 1.17 and 1.21, respectively, suggesting that both FTTCN- and FTTCN-M-based PSCs showed low trap-assisted recombination and the fluorene-fused ladder-type compound could be a good donor core for non-fullerene small molecule acceptors.
Plight (Fig. 3c), where k, T, and e are the Boltzmann constant, the temperature in Kelvin, and the elementary charge, respectively. It is well known that n is close to 1, implying that bimolecular recombination is the dominant mechanism; when n is close to 2, the additional mechanism of monomolecular recombination or trap-assisted recombination is involved. The dependences of VOC on Plight for the PBDB-T:FTTCN- and PBDB-T:FTTCN-M-based PSCs exhibited low slopes of 1.17 and 1.21, respectively, suggesting that both FTTCN- and FTTCN-M-based PSCs showed low trap-assisted recombination and the fluorene-fused ladder-type compound could be a good donor core for non-fullerene small molecule acceptors.
Photoluminescence (PL) quenching experiments
The photoluminescence (PL) quenching experiments were conducted to illustrate the exciton dissociation and charge transfer behavior in PBDB-T:FTTCN- and PBDB-T:FTTCN-M-based blends, where the excitation wavelengths are 530 nm for PBDB-T and 700 nm for FTTCN and FTTCN-M. As shown in Fig. 4, for the neat films, the PL emission peaks of FTTCN and FTTCN-M appear in the range of 740–900 nm. When FTTCN and FTTCN-M were blended with PBDB-T, the PL spectra were effectively quenched (92.3% for PBDB-T:FTTCN and 90.4% for PBDB-T:FTTCN-M). The high quenching efficiencies of PBDB-T:FTTCN and PBDB-T:FTTCN-M indicate that the blend films exhibit efficient photo-induced charge transfer between PBDB-T and the acceptors in the PSCs, which is consistent with the high JSCs in the PSCs. The PL emission peak of PBDB-T emerges in the range of 600–900 nm. The PL emission peaks of the blend films were highly quenched due to the effective electron transfer from PBDB-T to FTTCN and FTTCN-M, with exciton quenching efficiencies of 86.3% for FTTCN and 83.3% for FTTCN-M. These results imply that the electron transfer from PBDB-T to FTTCN/FTTCN-M is efficient.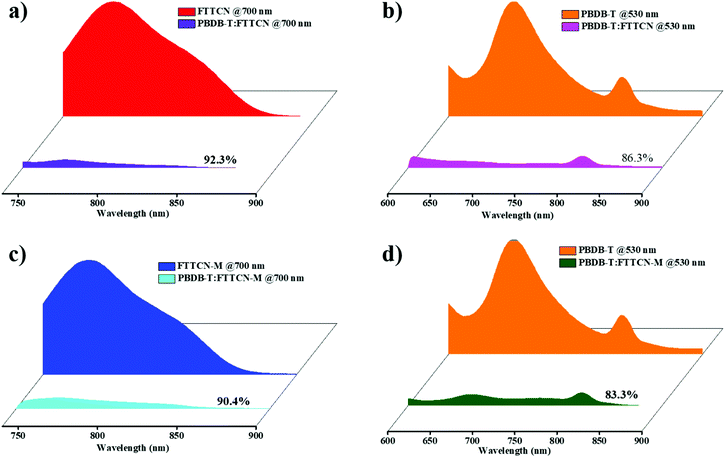 | ||
| Fig. 4 Photoluminescence spectra of (a and b) pure FTTCN and PBDB-T as well as PBDB-T:FTTCN blend films, and (c and d) pure FTTCN-M and PBDB-T as well as PBDB-T:FTTCN-M blend films. | ||
Hole and electron mobilities
In addition to the exciton dissociation and collection behaviors, carrier mobility also plays an important role in the photovoltaic performance of PSCs. The charge mobilities of the optimal blend films were measured by using the space charge limited current (SCLC) method. Hole-only and electron-only devices were fabricated with the structure of ITO/PEDOT:PSS/active layers/MoO3/Ag and ITO/ZnO/active layers/PDIN/Al, respectively (Fig. S5, ESI†). The hole and electron mobilities for the PBDB-T:FTTCN device and the PBDB-T:FTTCN-M device were 8.12 × 10−4/6.60 × 10−4 cm2 V−1 s−1 and 7.49 × 10−4/5.50 × 10−4 cm2 V−1 s−1, respectively (Table S3, ESI†). It was found that the FTTCN-based blend film achieved simultaneously enhanced electron and hole mobilities and more balanced charge transport with μh/μe being 1.23, which is smaller than that of the FTTCN-M based blend film (μh/μe = 1.35), indicating that CPTCN as the ending group is more beneficial to carrier transport in the active layer. Thus, a higher FF and a larger JSC could be obtained in PBDB-T:FTTCN based PSCs.Morphological studies
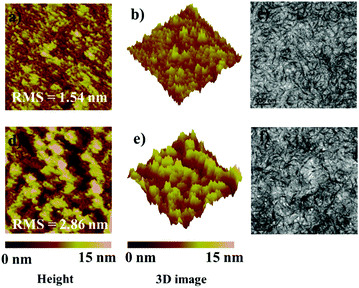
Considering the crucial relationship between morphology of blend film and device performance, atomic force microscopy (AFM) and transmission electron microscopy (TEM) measurements were conducted. As shown in Fig. 5, both PBDB-T:FTTCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) and PBDB-T:FTTCN-M (1
1 w/w) and PBDB-T:FTTCN-M (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) blend films exhibited quite smooth and uniform surfaces in the AFM height images. The root-mean-square (RMS) surface roughness was found to be 1.54 nm for PBDB-T:FTTCN and 2.86 nm for PBDB-T:FTTCN-M, indicating a more rough surface morphology for PBDB-T:FTTCN-M, which can be seen clearly from the 3D images of the blend films. The better morphological behavior of the PBDB-T:FTTCN blend film is more beneficial to the effective contacts between the PBDB-T:FTTCM active layer and electrode. Moreover, as can be seen in the AFM phase images (Fig. S6, ESI†), both the blend films exhibited obvious nanofiber microstructures, which are favorable for charge transport. From the TEM images, proper phase separation features can be observed in both PBDB-T:FTTCN- and PBDB-T:FTTCN-M-based blend films, which is beneficial for effective exciton dissociation.
1 w/w) blend films exhibited quite smooth and uniform surfaces in the AFM height images. The root-mean-square (RMS) surface roughness was found to be 1.54 nm for PBDB-T:FTTCN and 2.86 nm for PBDB-T:FTTCN-M, indicating a more rough surface morphology for PBDB-T:FTTCN-M, which can be seen clearly from the 3D images of the blend films. The better morphological behavior of the PBDB-T:FTTCN blend film is more beneficial to the effective contacts between the PBDB-T:FTTCM active layer and electrode. Moreover, as can be seen in the AFM phase images (Fig. S6, ESI†), both the blend films exhibited obvious nanofiber microstructures, which are favorable for charge transport. From the TEM images, proper phase separation features can be observed in both PBDB-T:FTTCN- and PBDB-T:FTTCN-M-based blend films, which is beneficial for effective exciton dissociation.
Conclusions
In summary, two newly developed SMAs based on the fluorene-fused electron-donating core FTT, namely FTTCN and FTTCN-M, were rationally designed and facilely synthesized. Compared to FT-based electron donors, the extended and coplanar conjugated structures of FTT-fused ladder-type SMAs can largely redshift the absorption spectrum and fine-tune the frontier orbital energy levels. PSCs based on PBDB-T:FTTCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) exhibited a maximum PCE of 10.56% with a VOC of 0.90 V, a JSC of 15.89 mA cm−2 and a FF of 73.82%. The higher JSC and FF for the FTTCN-based device can be attributed to its redshifted absorption, lower bimolecular recombination, better morphological behavior, higher charge mobility and more balanced charge transport. More importantly, this work manifested that a fluorene-fused ladder-type donor could be a promising building block for high-performance non-fullerene SMAs.
1, w/w) exhibited a maximum PCE of 10.56% with a VOC of 0.90 V, a JSC of 15.89 mA cm−2 and a FF of 73.82%. The higher JSC and FF for the FTTCN-based device can be attributed to its redshifted absorption, lower bimolecular recombination, better morphological behavior, higher charge mobility and more balanced charge transport. More importantly, this work manifested that a fluorene-fused ladder-type donor could be a promising building block for high-performance non-fullerene SMAs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) (No. 21572171) and the Innovative Research Group of Hubei Province (No. 2015CFA014).Notes and references
- G. Yu, J. Cao, J. C. Hummelen, F. Wuld and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 RSC.
- L. Dou, Y. Liu, Z. Hong, G. Li and Y. Yang, Chem. Rev., 2015, 115, 12633–12665 CrossRef CAS PubMed.
- B. Thompson and J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397–7457 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- C. Nielsen, S. Holliday, H. Chen, S. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803–2812 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- C.-C. Chen, W.-H. Chang, K. Yoshimura, K. Ohya, J. You, J. Gao, Z. Hong and Y. Yang, Adv. Mater., 2014, 26, 5670–5677 CrossRef CAS PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174–179 CrossRef CAS.
- H. Zhou, Y. Zhang, C.-K. Mai, S. D. Collins, G. C. Bazan, T.-Q. Nguyen and A. J. Heeger, Adv. Mater., 2015, 27, 1767–1773 CrossRef CAS PubMed.
- T. Liu and A. Troisi, Adv. Mater., 2013, 25, 1038–1041 CrossRef CAS PubMed.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- C. Lee, H. Kang, W. Lee, T. Kim, K. Kim, H. Y. Woo, C. Wang and B. J. Kim, Adv. Mater., 2015, 27, 2466–2471 CrossRef CAS PubMed.
- Q. Wu, D. Zhao, A. M. Schneider., W. Chen and L. Yu, J. Am. Chem. Soc., 2016, 138, 7248–7251 CrossRef CAS PubMed.
- D. Meng, H. Fu, C. Xiao, X. Meng, T. Winands, W. Ma, W. Wei, B. Fan, L. Huo, N. L. Doltsinis, Y. Li, Y. Sun and Z. Wang, J. Am. Chem. Soc., 2016, 138, 10184–10190 CrossRef CAS PubMed.
- E. Zhou, J. Cong, Q. Wei, K. Tajima, C. Yang and K. Hashimoto, Angew. Chem., Int. Ed., 2011, 50, 2799–2803 CrossRef CAS PubMed.
- B. Fan, L. Ying, Z. Wang, B. He, X. Jiang, F. Huang and Y. Cao, Energy Environ. Sci., 2017, 10, 1243–1251 RSC.
- Z. Luo, T. Liu, W. Cheng, K. Wu, D. Xie, L. Huo, Y. Sun and C. Yang, J. Mater. Chem. C, 2018, 6, 1136–1142 RSC.
- Y. Lin, J. Wang, Z. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- Y. Li, J. Lin, X. Che, Y. Qu, F. Liu, L. Liao and S. R. Forrest, J. Am. Chem. Soc., 2017, 139, 17114–17119 CrossRef CAS PubMed.
- W. Gao, Q. An, R. Ming, D. Xie, K. Wu, Z. Luo, Y. Zou, F. Zhang and C. Yang, Adv. Funct. Mater., 2017, 27, 1702194 CrossRef.
- Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955–4961 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganas, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734–4739 CrossRef CAS PubMed.
- H. Bin, Z. G. Zhang, L. Gao, S. Chen, L. Zhong, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 4657–4664 CrossRef CAS PubMed.
- Z. Luo, H. Bin, T. Liu, Z. Zhang, Y. Yang, C. Zhong, B. Qiu, G. Li, W. Gao, D. Xie, K. Wu, Y. Sun, F. Liu, Y. Li and C. Yang, Adv. Mater., 2018, 30, 1706124 CrossRef PubMed.
- M. Zhang, Z. Xiao, W. Gao, Q. Liu, K. Jin, W. Wang, Y. Mi, Q. An, X. Ma, X. Liu, C. Yang, L. Ding and F. Zhang, Adv. Energy Mater., 2018, 1801968 CrossRef.
- W. Liu, J. Zhang, Z. Zhou, D. Zhang, Y. Zhang, S. Xu and X. Zhu, Adv. Mater., 2018, 30, 1800403 CrossRef PubMed.
- S. Zhang, Y. Qin, J. Zhu and J. Hou, Adv. Mater., 2018, 30, 1800868 CrossRef PubMed.
- S. Li, L. Ye, W. Zhao, H. Yan, B. Yang, D. Liu, W. Li, H. Ade and J. Hou, J. Am. Chem. Soc., 2018, 140, 7159–7167 CrossRef CAS PubMed.
- J. Sun, X. Ma, Z. Zhang, J. Yu, J. Zhou, X. Yin, L. Yang, R. Geng, R. Zhu, F. Zhang and W. Tang, Adv. Mater., 2018, 30, 1707150 CrossRef PubMed.
- H. Zhang, H. Yao, J. Hou, J. Zhang, W. Li, R. Yu, B. Gao, S. Zhang and J. Hou, Adv. Mater., 2018, 30, 1800613 CrossRef PubMed.
- D. He, F. Zhao, J. Xin, J. J. Rech, Z. Wei, W. Ma, W. You, B. Li, L. Jiang, Y. Li and C. Wang, Adv. Energy Mater., 2018, 8, 1802050 CrossRef.
- D. Yan, W. Liu, J. Yao and C. Zhan, Adv. Energy Mater., 2018, 8, 1800204 CrossRef.
- R. Yu, H. Yao, L. Hong, Y. Xu, B. Gao, J. Zhu, Y. Zu and J. Hou, Adv. Energy Mater., 2018, 8, 1802131 CrossRef.
- Q. Fan, Y. Wang, M. Zhang, B. Wu, X. Guo, Y. Jiang, W. Li, B. Guo, C. Ye, W. Su, J. Fang, X. Ou, F. Liu, Z. Wei, T. C. Sum, T. P. Russell and Y. Li, Adv. Mater., 2018, 30, 1704546 CrossRef PubMed.
- L. Xue, Y. Yang, J. Xu, C. Zhang, H. Bin, Z.-G. Zhang, B. Qiu, X. Li, C. Sun, L. Gao, J. Yao, X. Chen, Y. Yang, M. Xiao and Y. Li, Adv. Mater., 2017, 29, 1703344 CrossRef PubMed.
- W. Gao, Q. An, C. Zhong, Z. Luo, R. Ming, M. Zhang, Y. Zou, F. Liu, F. Zhang and C. Yang, Chem. Sci., 2018, 9, 8142–8149 RSC.
- W. Liu, Z. Zhou, T. Vergote, S. Xu and X. Zhu, Mater. Chem. Front., 2017, 1, 2349–2355 RSC.
- N. Wang, L. Zhan, S. Li, M. Shi, T. Lau, X. Lu, R. Shikler, C. Li and H. Chen, Mater. Chem. Front., 2018, 2, 2006–2012 RSC.
- J. Lee, S. Ko, M. Seifrid, H. Lee, B. R. Luginbuhl, A. Karki, M. Ford, K. Rosenthal, K. Cho, T. Nguyen and G. C. Bazan, Adv. Energy Mater., 2018, 8, 1801212 CrossRef.
- N. Qiu, H. Zhang, X. Wan, C. Li, X. Ke, H. Feng, B. Kan, H. Zhang, Q. Zhang, Y. Lu and Y. Chen, Adv. Mater., 2017, 29, 1604964 CrossRef PubMed.
- Z. Zhang, M. Li, Y. Liu, J. Zhang, S. Feng, X. Xu, J. Song and Z. Bo, J. Mater. Chem. A, 2017, 5, 7776–7783 RSC.
- Z. Luo, C. Sun, S. Chen, Z. Zhang, K. Wu, B. Qiu, C. Yang, Y. Li and C. Yang, Adv. Energy Mater., 2018, 8, 1800856 CrossRef.
- Z. Li, X. Xu, W. Zhang, X. Meng, W. Ma, A. Yartsev, O. Inganäs, M. R. Andersson, R. A. J. Janssen and E. Wang, J. Am. Chem. Soc., 2016, 138, 10935–10944 CrossRef CAS PubMed.
- Z. Li, X. Xu, W. Zhang, X. Meng, Z. Genene, W. Ma, W. Mammo, A. Yartsev, M. R. Andersson, R. A. J. Janssen and E. Wang, Energy Environ. Sci., 2017, 10, 2212–2221 RSC.
- D. J. Wehenkel, K. H. Hendriks, M. M. Wienk and R. A. J. Janssen, Org. Electron., 2012, 13, 3284–3290 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qm00013e |
| This journal is © the Partner Organisations 2019 |