Laser-induced synthesis of ZIF-67: a facile approach for the fabrication of crystalline MOFs with tailored size and geometry†
Abstract
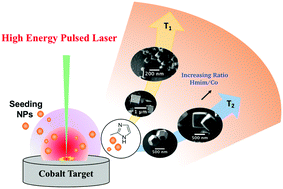
Metal organic frameworks (MOFs) are fast emerging as a new class of crystalline hybrid porous materials originating from inorganic (metal) nodes connected by organic linkers. MOFs have gained tremendous interest in the materials science community owing to their versatile functionalities as a result of tailorable chemical activity, porosity, and morphology. In spite of the wide classes of MOF structures synthesized in recent years, rapid yet environmentally friendly fabrication strategies that allow precise morphological and structural control of these frameworks is still challenging. Herein, we report a facile route using high-energy laser ablation synthesis in solution (LASiS) to produce highly crystalline zeolitic imidazolate framework-67 (ZIF-67) structures by ablating Co targets in 2-methylimidazole (Hmim) solutions. Synthesis protocols presented here demonstrate for the first time the ability of LASiS to rationally tailor the sizes and shapes of MOF structures by adjusting solution-phase (reagent concentration and temperature) and laser parameters (ablation time). Our findings indicate that while the average MOF size is controlled by varying the organic linker to LASiS-generated metal ion stoichiometric ratio, tailoring of its morphology occurs via precise control of synthesis temperature and duration of ablation. Finally, a mechanistic picture for MOF formation via LASiS is presented.


 Please wait while we load your content...
Please wait while we load your content...