Luminescent solar concentrators: boosted optical efficiency by polymer dielectric mirrors†
G.
Iasilli
a,
R.
Francischello
a,
P.
Lova
 b,
S.
Silvano
b,
A.
Surace
b,
G.
Pesce
b,
M.
Alloisio
b,
M.
Patrini
c,
M.
Shimizu
b,
S.
Silvano
b,
A.
Surace
b,
G.
Pesce
b,
M.
Alloisio
b,
M.
Patrini
c,
M.
Shimizu
 d,
D.
Comoretto
d,
D.
Comoretto
 *b and
A.
Pucci
*b and
A.
Pucci
 *a
*a
aDipartimento di Chimica e Chimica Industriale, Università di Pisa, via Moruzzi 13, 56124 Pisa, Italy. E-mail: davide.comoretto@unige.it
bDipartimento di Chimica e Chimica Industriale, Università di Genova, Via Dodecaneso 31, 16146 Genova, Italy. E-mail: andrea.pucci@unipi.it
cDipartimento di Fisica, Università di Pavia, via Bassi 6, 27100 Pavia, Italy
dFaculty of Molecular Chemistry and Engineering, Kyoto Institute of Technology, 606-8585 Kyoto, Japan
First published on 4th January 2019
Abstract
We report on the optical efficiency enhancement of luminescent solar concentrators based on a push–pull fluorophore realized using high dielectric contrast polymer distributed Bragg reflectors as back mirrors. The Bragg stacks are obtained by alternating layers of cellulose acetate and thin films of a new stable and solution processable hydrated titania–poly(vinyl alcohol) nanocomposite (HyTiPVA) with a refractive index greater than 1.9 over a broad spectral range. The results obtained with these systems are compared with enhancements provided by standard Bragg reflectors made of commercial polymers. We demonstrate that the application of the Bragg stacks with photonic band-gap tuned to the low energy side of the dye emission spectrum induces a 10% enhancement of optical efficiency. This enhancement is the result of a photon recycling mechanism and is retained even in a scaled-up device where the Bragg mirrors are used in a mosaic configuration.
Introduction
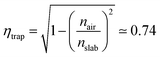
Nowadays, cost reduction and efficiency enhancement are the driving forces for technological development of photovoltaic (PV) systems.1 In recent years, luminescent solar concentrators (LSCs) have become appealing thanks to their light weight, high concentration factors, and the possibility of operating with diffuse light without the need for expensive solar trackers and coolers.2 Moreover, these devices can be easily integrated into modern constructions and, together with other systems for energy saving, such as adaptive windows,3,4 could allow zero energy consumption buildings, accordingly to the EU guideline 2010/31/UE for 2020.Even though LSCs are already available on the market,5 some drawbacks are still limiting their massive commercial distribution. Such drawbacks include difficulties in the preparation of easily mountable modules and in the improvement of the device efficiency, which can be understood by analyzing their working principle. LSCs are highly transparent, planar and relatively thick waveguides doped with high quantum yield fluorophores.2 The slabs have a refractive index higher than their surroundings. In this way they favor total internal reflection of light emitted within the slab and its guiding to its sides, where standard solar cells are placed.2 Notwithstanding their simplicity, several processes rule and limit their global device efficiency (ηdev), including the usually poor matching between the fluorophore absorption spectrum and the solar emission (ηABS) as well as the dye emission efficiency (ηPL). Besides the issues related to the fluorophore, the efficiency of the lateral solar cells (ηPV), the waveguiding process (ηWG), and the trapping process (ηtrap) affect the entire energy generation process such that:
| ηdev = ηABSηPLηWGηPVηtrap. | (1) |
 | (2) |
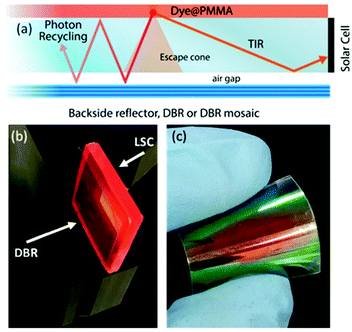 | ||
| Fig. 1 (a) Schematic of the LSC configuration and main processes involved. Digital photograph of (b) the LSC device coupled to a DBR and (c) of a flexible DBR. | ||
In this work, we report on the role of polymer distributed Bragg reflectors (DBR) as back mirror – in place of a standard diffuser – on the performances of LSCs (Fig. 1(b and c)). Polymer DBRs and related structures with very high reflectance in a limited spectral region have been already exploited for lasing, fluorescence emission control, optical switches, and sensors.29–37 The optical responses of DBR, including the spectral position of the photonic band-gap, its reflectance intensity and bandwidth, are mainly dictated by the periodicity of the structure and the refractive index contrast among the polymer components.36 Here, in order to increase the reflection bandwidth, we spun-cast high dielectric contrast polymer DBRs properly tuned to enhance the LSC performances. The DBRs allowed a ∼10% enhancement of the optical efficiency that is retained also on scaled-up devices through mosaicking of the DBRs. To this end, we employed both polymer DBRs fabricated alternating commercial cellulose acetate (CA) and poly(N-vinyl carbazole) (PVK) layers (sample series P), or CA and the novel processable hydrated titania–poly(vinyl alcohol) nanocomposites (HyTiPVA) with a very high refractive index (sample series H).
Experimental section
Fluorophore synthesis and characterization
SilaFluo was synthesized according to the literature.17,38 Absorption and reflectance spectra were measured at room temperature by an Agilent Cary5000 UV-Vis-NIR spectrophotometer equipped with an Internal Diffuse Reflectance DRA-2500. Fluorescence spectra were measured at room temperature by a Horiba Jobin-Yvon Fluorolog®-3 spectrofluorometer equipped with a 450 W Xenon arc lamp and single and double grating excitation and emission monochromators, respectively.LSC preparation
To prepare the fluorophore–PMMA layer, about 30 mg of PMMA and SilaFluo were dissolved in ∼0.8 mL of chloroform and stirred for 30 min at room temperature. Subsequently, the solution was spread out evenly on a thoroughly cleaned 35 × 50 mm glass surface to obtain a film with thickness 25 ± 5 μm (Starrett micrometer) after evaporation at room temperature in a closed environment. The polymer film was then removed after immersion in water and stored in a desiccator for successive measurements by attaching them on 24 × 24 × 3 mm (geometrical factor, G = 8) or 50 × 50 × 3 mm (G = 16.7) cleaned glass (Edmund Optics Ltd BOROFLOAT window) with a high-purity silicone oil (poly(methylphenyl siloxane), 710 fluid, Aldrich, n = 1.5365) layer. The diffuser and the DBRs or DBR mosaic (4 DBRs) were placed beneath the LSC with G = 8 (G = 16.7).Preparation of HyTiPVA
The HyTiPVA composite was prepared by mixing aqueous solutions of PVA and HyTi with different concentrations adapting a wet synthetic protocol previously reported.39 HyTi solutions were previously obtained through a controlled hydrolysis of commercial TiCl4 (Sigma-Aldrich, purity >99%) by slow addition of 8 mL of TiCl4 cooled at 0 °C with ice to 62.5 mL of water. The mixtures were maintained under constant stirring at room temperature for 12 h to ensure full reaction. A clear colorless HyTi solution with a Ti concentration of 1.03 mmol L−1 was obtained. To produce the hybrid material, the freshly prepared HyTi solutions were added to a 20 g L−1 aqueous solution of PVA (Sigma-Aldrich, (〈Mn〉 = 1.66 × 105 g mol−1, 99+% hydrolyzed) at a constant ratio of 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The samples were transparent in the vis-NIR spectral interval (Fig. S1, ESI†) and solution processable for the preparation of spin-coated films. For this purpose, the filmability of hybrid solutions was optimized by the addition of EtOH in the ratio of 1
1 v/v). The samples were transparent in the vis-NIR spectral interval (Fig. S1, ESI†) and solution processable for the preparation of spin-coated films. For this purpose, the filmability of hybrid solutions was optimized by the addition of EtOH in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v before the mixture deposition.
2 v/v before the mixture deposition.
Polymer DBRs
P series DBRs were prepared by spin-coating CA (Aldrich, Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) dissolved in diacetone alcohol (35 mg mL−1) and PVK (ACROS Organic, Mn = 56
000) dissolved in diacetone alcohol (35 mg mL−1) and PVK (ACROS Organic, Mn = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Mw = 135
400 Mw = 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) in toluene solutions (28 mg mL−1) on poly(ethylene terephthalate) (PET) substrates; the rotation speed was kept between 80 and 105 RPS. H series DBRs were prepared by casting alternate layers of HyTiPVA and the CA solution on glass substrates with rotation speed ranging between 80 and 120 RPS. More details are reported in Table S1 (ESI†).
600) in toluene solutions (28 mg mL−1) on poly(ethylene terephthalate) (PET) substrates; the rotation speed was kept between 80 and 105 RPS. H series DBRs were prepared by casting alternate layers of HyTiPVA and the CA solution on glass substrates with rotation speed ranging between 80 and 120 RPS. More details are reported in Table S1 (ESI†).
Optical efficiency of LSCs
The optical efficiency of the LSC was measured with a home-built equipment setup. Each DBR, single or mosaic, was placed beneath the LSC of G = 8 or G = 16, respectively. Each sample was tested in triplicate. A solar simulating lamp (ORIEL® LCS-100 solar simulator 94011A S/N: 322, AM 1.5G std filter: 69 mW cm−2 at 254 mm) was housed 27.5 cm above the sample. The PV module (IXYS SLMD121H08L mono solar cell 86 × 14 mm) was connected to a digital potentiometer (AD5242) controlled via I2C by an Arduino Uno micro-controller using I2C master library.40 A digital multimeter (KEITHLEY 2010) was connected in series with the circuit, between the photovoltaic module and the potentiometer, to collect the current as a function of the external load. Conversely, the voltage was measured by connecting the multimeter in parallel to the digital potentiometer.Optical function characterization
Spectroscopic ellipsometry measurements have been performed on reference thin films of the different materials, by using a VASE instrument by J. A. Woollam Co. in the range 250–2500 nm at different angles of incidence from 60° to 75°.41 Transmittance at normal incidence has also been measured with a Varian Cary 6000i spectrometer in the spectral range 300–1800 nm. As a result, the complex refractive index n + ik for all materials was evaluated by WVASE32® software, adopting oscillator models that guarantee Kramers–Kronig consistency and effective-medium approximation for the HyTiPVA nanocomposite.Results and discussion
The standard LSC devices were fabricated casting a thin layer of poly(methyl methacrylate) (PMMA) doped with a SilaFluo fluorophore on a glass slab. Then, a diffuser layer was applied to the back of the slab with an air gap (Fig. 1, see Fig. S1 (ESI†) for the optical characterization of the diffuser and the slab). As mentioned before, the air gap guarantees that the slab guiding properties are maintained. This system represents the reference LSC. In our improved LSC devices, the diffuser was replaced with different Bragg stacks maintaining the air gap, as described in the Experimental section.The fluorophore used in this work is a red-emitting 2-amino-7-acceptor-9-silafluorene, where the amino group–N(CH3)2 is the donor, and the acceptor is –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2 (SilaFluo, Fig. 2(a)). This dye shows a fluorescence quantum yield of 65% and has already been successfully used in high performance LSCs.17,22Fig. 2(b) shows the absorbance and fluorescence spectra of the 1.5 wt% SilaFluo embedded in the PMMA film and compares them with the transmittance spectrum normalized to a bare PMMA film. Notwithstanding the absorbance of SilaFluo that overlaps the solar emission spectrum only partially, limiting ηABS, it shows a relatively large Stokes shift. Indeed, while the absorption peak is positioned at 478 nm, the fluorescence is centered at 620 nm, limiting re-absorption losses which commonly affect ηPL. Moreover, SilaFluo is stable under LSC working conditions and provides an excellent matching with the spectral response of the side Si-solar cells.
C(CN)2 (SilaFluo, Fig. 2(a)). This dye shows a fluorescence quantum yield of 65% and has already been successfully used in high performance LSCs.17,22Fig. 2(b) shows the absorbance and fluorescence spectra of the 1.5 wt% SilaFluo embedded in the PMMA film and compares them with the transmittance spectrum normalized to a bare PMMA film. Notwithstanding the absorbance of SilaFluo that overlaps the solar emission spectrum only partially, limiting ηABS, it shows a relatively large Stokes shift. Indeed, while the absorption peak is positioned at 478 nm, the fluorescence is centered at 620 nm, limiting re-absorption losses which commonly affect ηPL. Moreover, SilaFluo is stable under LSC working conditions and provides an excellent matching with the spectral response of the side Si-solar cells.
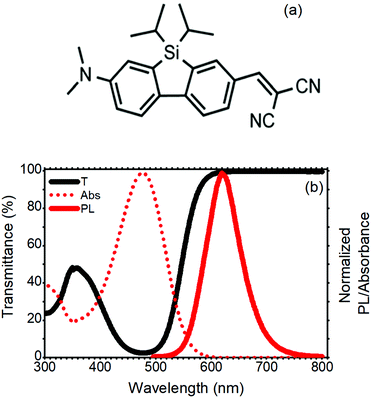 | ||
| Fig. 2 (a) SilaFluo chemical structure. (b) Transmittance and normalized absorbance and photoluminescence spectra of the 1.5 wt% silaFluo–PMMA film. | ||
Two series of DBRs were fabricated with the CA–PVK and CA–HyTiPVA pairs tuning their PBGs in different spectral regions of the fluorophore emission. Then, the DBRs were placed on the back side of the LSC with the aim to reflect photons leaving the slab from the escape cone (see Fig. 1(a)). To obtain the best performances from the DBRs, their PBGs should be spectrally tuned to the low energy side of the fluorophore emission and should have a large full width at half maximum (FWHM).27,28 First, the spectral tuning and the angle of incidence dispersion of the PBG of the DBR allow the mirrors to work finely for all incidence geometry, i.e. for any daily sun illumination conditions.9,36,42 Second, a PBG FWHM larger than the dye fluorescence spectrum is desirable to reflect all the light escaping from the slab. Both the PBG spectral tuning and width are mainly dictated by the periodicity and the dielectric contrast among the DBR components.36 In more detail, the PBG position is commonly controlled by engineering the layer thicknesses, while its spectral width is only dictated by the dielectric contrast of the materials used. Large dielectric contrast inorganic DBR structures usually perform best,28 while commodity polymers provide reduced dielectric contrast, but allow very light and flexible mirrors that can be fabricated even on the square meter area (Fig. 1(c)).36,43–45 To increase the dielectric contrast in polymer structures, several issues mainly due to the constraint of mutual processability have to be addressed.31,32 Indeed, developing suitable high index systems is not straightforward, while the use of low refractive index polymers suitable for solution growth of DBRs is very complex.35,39,46–48 Only two strategies, which show relevant drawbacks, have been reported so far. For instance, highly porous polymers have very low refractive index,49,50 but their high void volume fraction prevents their use for the fabrication of DBRs due to percolation of the high index counterpart within the porosity. Low refractive index perfluorinated polymers have been instead successfully employed to spun-cast DBRs,35,47 but the cost of such materials is very high and their processability requires specific know-how to allow fine spectral tuning and surface wettability. For these reasons, we decided to use CA as the low refractive index material for DBR fabrication; in fact, it is widely employed and easily processable.36 The refractive index of CA is about 1.47 over a broad spectral region (black line in Fig. 3(a)). In this range, the polymer thin film does not show absorption bands assigned to electronic transitions, which makes it highly suitable as a transparent material for DBR fabrication. In P series DBR, we coupled CA to PVK, which shows relevant absorption of below 300 nm and a refractive index value of about 1.67 (green line in Fig. 3(a)). Indeed, CA and PVK have often been coupled in the literature for the fabrication of polymer DBR for different applications.36 Currently, PVK is the solution-processable polymer with the highest refractive index over a very broad spectral range available commercially.51–54 However, coupling CA and PVK does not allow us to achieve dielectric contrast higher than 0.21, thus limiting the PBG width. Moreover, a very large number of periods are necessary to achieve reflectance values close to unity.32,36,46
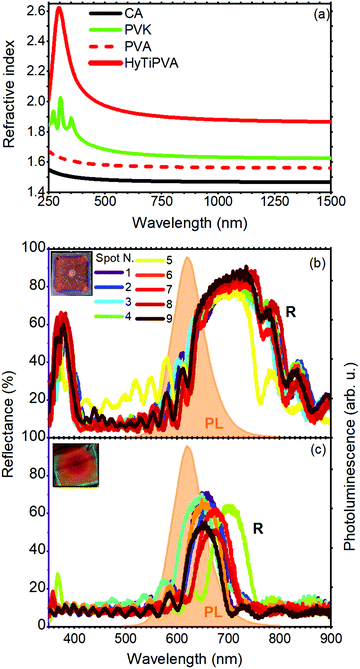 | ||
| Fig. 3 (a) Refractive index of CA (black line) and PVK (green line) from the literature,41,51,52 PVA (red dashed line) and HyTiPVA (red continuous line) as determined from ellipsometry measurements. (b and c) Reflectance spectra over nine different positions of the polymer DBRs made by CA–HyTiPVA and CA–PVK, respectively. In the same panels, the photoluminescence spectrum of SilaFluo is shown as dashed orange area, while the insets show the digital photographs of the samples. | ||
One of the most promising strategies to achieve a high refractive index in polymer matrices consists in the loading of high refractive index nanofillers such as titania nanoparticles (n = 2.5).55 To significantly increase the complex refractive index (ñ) in nanocomposites suitable for photonics, two requirements are mandatory. First, large nanofiller volume fractions are needed. Second, a very small size of nanoparticles and no tendency to aggregation are necessary to prevent light scattering and maintain device transparency. The combination of these requirements, along with the need for high solution processability, makes this approach challenging.56 We developed a new processable material with the refractive index higher than that of PVK. To this end, we refined a method previously reported to significantly increase the refractive index of PVA, grafting hydrated titania directly to the hydroxylic group of the polymer.39,57 PVA is indeed particularly appealing owing to the large amounts of hydroxylic substituents, which can be used as grafting sites for the nanofiller, thus acting as spacers, drastically reducing the aggregation processes and eliminating the need for surfactants (see Experimental section).58,59 We then used the new HyTiPVA and CA to spin-cast a series (H) of high performance DBRs with PBG easily tunable on the emission spectrum of the LSC fluorophore. The optical response of the new HyTiPVA material was determined by spectroscopic ellipsometry, and the real part (n) of the complex refractive index (ñ = n + ik) is shown in Fig. 3(a) and compared with other polymers used in this work. The loading resulted in a dramatic increase of the PVA refractive index. Indeed, while bare PVA showed a refractive index of about 1.55 in the analyzed spectral range (red dashed line in Fig. 3(a)), after the loading of HyTi, the index approached 1.9 over the entire near infrared and visible spectral regions (red line in Fig. 3(a)). The full spectral response of ñ is shown in Fig. S2 (ESI†). From the spectrum reported in Fig. 3(a), according to a simple Maxwell–Garnett effective medium model55 and considering the refractive index of the HyTiPVA equal to the one of anatase TiO2, we estimated a volume fraction load of at least 30% Moreover, no absorption due to electronic transition was detected in the sample spectral range (see also Fig. S2, ESI†). These characteristics, together with the good processability of PVA, make the new composite a promising high refractive index medium to be coupled with CA.
The high refractive index of the HyTiPVA hybrid has a remarkable effect on the PBG FWHM. Fig. 3(b) and (c) compares the reflectance spectra of two DBRs made of CA and the high refractive index polymers (HyTiPVA, sample H1 in panel b; PVK, sample P1 in panel c). The reflectance spectra of the sample H1 measured in nine different spots of the sample surface show a large reflectance peak centered at 750 nm with a FWHM of 170 nm, followed by a second order peak centered at 377 nm (Fig. 3(b), more spectral information and photographs are shown in Fig. S3, ESI†). Due to the deposition process, the central spot of the sample surface (spot N. 5) commonly differs from the others, affecting the surface homogeneity.29,60 On the other hand, the good overlap of the other spectra, together with the interference pattern, testifies the homogeneity and the good optical quality of the sample. The presence of the second order PBG indicates that the mirrors do not fulfill the lambda fourth condition often used for laser cavities,36 thus possibly allowing a wider FWHM. The background provides an average reflectance of about 10%. Comparing the reflectance spectra of the H1 DBR to the LSC emission and transmittance (Fig. 2(b), the emission spectrum is also highlighted in orange in Fig. 3(b) and (c)), we notice the tuning of the first order PBG in the emission spectral region and to its low energy side. DBRs with PBG tuned in different regions have also been fabricated and tested as reported in Fig. S4 (ESI†) for samples H2–H8.
The CA–PVK DBR is instead characterized by a first order PBG at 660 nm with a FWHM of 70 nm, positioned on the low energy side of the fluorophore emission (Fig. 3(c)). The second order PBG in this case has a very low intensity and is slightly visible only in two of the nine spots measured, demonstrating that the sample fulfills the lambda fourth condition.36 More spectral information and images of this sample are reported in Fig. S5 (ESI†). Comparing the spectra of Fig. 3(b and c), we notice that the CA–PVK sample is less homogeneous than the one fabricated using the HyTiPVA nanocomposite. Moreover, the PBG intensity and width are smaller than for the CA–HyTiPVA DBR but, as shown in the following, this sample provides the best performances when applied to the LSC. Fig. S6 (ESI†) shows the optical characterization of the other samples of the series (P2–P8).
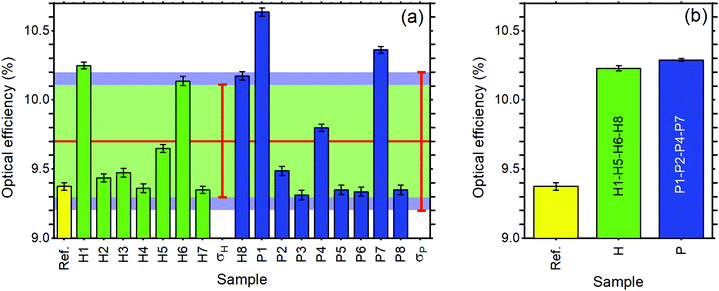
Regarding the performance of the SilaFluo–LSCs, we first focus on devices of size 24 × 24 × 3 mm3. These LSCs have a geometrical factor, i.e. the ratio between the illuminated surface area and the solar cell area, of G = 8. As described before, a diffuser layer is mounted on the back of the reference LSC with an air gap to prevent propagation losses (constant ηWG). To assess the DBR effect on the LSC performances, we used optical efficiency (ηLSCopt) of the side cells integrated spectrally:61,62
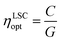 | (3) |
For the reference LSC, we found an optical efficiency of 9.4% (Fig. 4) with C = 0.75, in full agreement with our recent findings.17 We then replaced the diffusing layer with CA–HyTiPVA (samples H). The new systems show optical efficiencies ranging from 9.4% to 10.3% with mean 9.7% and standard deviation σ = 0.4%, i.e. up to a 10% enhancement factor. When the diffuser is replaced with the P series of DBRs (CA–PVK), the optical efficiency of the devices is more heterogeneous and ranges from 9.3%, which is lower than the reference efficiency, to 10.6%, which represents the best enhancement achieved, the mean value achieved being 9.7% with σ = 0.5%.
The better homogeneity of the data obtained for the H series can be explained by considering the PBG reflectance intensity and FWHM of the two systems. For the H series, the higher dielectric contrast with respect to the samples prepared with PVK allows wider PBGs and in turn their overlapping to the largest part of the fluorophore emission spectrum, even for different PBG tuning, making the H series very efficient reflectors for photons leaving the slab within the escape cone (Fig. 1). Then, notwithstanding possible tuning errors and a low PBG reflectance value of some of the samples at the PBG (see for instance sample H7 in Fig. S4, ESI†), all the samples prepared with the HyTiPVA composite perform better than the reference one with the diffuser. In particular, those samples tuned on the low energy side of fluorophore fluorescence and with a high FWHM (H1, H6, H8) provide the best enhancements of optical efficiency due to photon recycling of light for angle of incidence far from the normal direction. Conversely, for CA–PVK DBRs of the series P, both the PBG reflectance intensity and FWHM are relatively low. This characteristic makes the efficiency of the photon recycling more sensitive to the spectral tuning of the photonic structure. These results demonstrate that either a high dielectric contrast or a fine tuning of the photonic structure is necessary to achieve a significant enhancement of the optical efficiency of LSCs using spun-cast polymer DBRs. We would like to stress that polymer DBRs show a substantial advantage over standard mirrors used for LSC. Indeed, these structures are much lighter, are easily adaptable to any surface (even curved if requested, Fig. 1(c)), and can be eventually grown by different techniques, such as coextrusion, over square meters at industrial level.36,43,44,63
To evaluate the scale-up opportunities of our approach, we also tested the DBRs in mosaic configuration on larger LSC, e.g. by doubling the LSC size (G = 16). In this case, we created a DBR mosaic coupling the larger LSC to 4 DBR mirrors. Fig. 4(b) shows that for the larger device when the diffuser is used, the device optical efficiency does not differ from the previous case. We then exchanged the diffuser with the four best performing DBRs for each of the two series, thus enhancing the efficiency to 10.2% and 10.3% for the H and P series, respectively. Such an enhancement, which corresponds to a ∼9.5% increase, is impressive considering the detrimental effects of the photonic structure edges, which are known to reduce the performance of LSCs.9 Again, the use of industrial techniques previously highlighted for large area DBR production could be of great help to scale up the dimension of LSCs, thus making them a widespread and successful technology.
Conclusions
We demonstrated that polymer DBRs made of commercial polymers including CA as the low index medium and PVK or HyTiPVA nanocomposite fabricated ad hoc by simple wet chemistry can enhance the optical efficiency of LSCs by up to a ∼10% when used as back reflectors with respect to the same system with a standard diffuser. Moreover, we proved that the enhancement is retained during the scale-up of the device area by a factor of 4 and using the DBR back reflectors in mosaic configuration. The transparency in the largest part of the visible spectral range of the LSC–DBR devices, together with the possibility to fabricate these systems on the square meter area using industrial techniques, paves the way to their application in integrated photovoltaic systems for zero energy consumption buildings in the near future.Author contributions
The project was conceived by D. C. and A. P. G. I. and R. F. fabricated the LSC devices and characterized their performances, P. L., S. S., A. S., and G. P. fabricated and characterized the DBR structures, M. P. performed the ellipsometric measurements, and M. S. synthetized the SilaFluo dye. Work in Genova was supervised by D. C. and M. A. A. P supervised the work in Pisa. The manuscript was written through contributions from all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Work in Genova is supported by the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement No. 643238. The authors also acknowledge support from the University of Genova and Pisa. The research leading to these results has received funding from the Università di Pisa under PRA 2017 (project No. 2017_28) and BIHO 2017 and from the University of Genova under PRA 2017.Notes and references
-
V. Balzani and N. Armaroli, Energy for a sustainable world, Wiley-VCH, Weinheim, 2010 Search PubMed
.
- M. G. Debije and P. P. C. Verbunt, Adv. Energy Mater., 2012, 2, 12–35 CrossRef CAS
.
-
Nanomaterials for Sustainable Energy, ed. Q. Li, Springer, Heidelberg, 1st edn, 2016 Search PubMed
.
- L. Wang, H. K. Bisoyi, Z. Zheng, K. G. Gutierrez-Cuevas, G. Singh, S. Kumar, T. J. Bunning and Q. Li, Mater. Today, 2017, 20, 230–237 CrossRef CAS
.
- G. Galloro, https://www.eniday.com/it/technology_it/eni-ray-plus-finestre-intelligenti/, https://www.eniday.com/it/technology_it/eni-ray-plus-finestre-intelligenti/, accessed 14/10/2018.
- F. Meinardi, H. McDaniel, F. Carulli, A. Colombo, K. A. Velizhanin, N. S. Makarov, R. Simonutti, V. I. Klimov and S. Brovelli, Nat. Nanotechnol., 2015, 10, 878 CrossRef CAS PubMed
.
- P. Moraitis, R. E. I. Schropp and W. G. J. H. M. van Sark, Opt. Mater., 2018, 84, 636–645 CrossRef CAS
.
- F. Meinardi, F. Bruni and S. Brovelli, Nat. Rev. Mater., 2017, 2, 17072 CrossRef CAS
.
- A. Bozzola, V. Robbiano, K. Sparnacci, G. Aprile, L. Boarino, A. Proto, R. Fusco, M. Laus, L. C. Andreani and D. Comoretto, Adv. Opt. Mater., 2016, 4, 147–155 CrossRef CAS
.
-
R. Fusco, L. C. Andreani, A. Bozzola, D. Comoretto, V. Robbiano, M. Laus and K. Sparnacci, Hybrid concentrated photovoltaic device, US Pat., US2018248063 (A1), 2018 Search PubMed
.
- F. Meinardi, A. Colombo, K. A. Velizhanin, R. Simonutti, M. Lorenzon, L. Beverina, R. Viswanatha, V. I. Klimov and S. Brovelli, Nat. Photonics, 2014, 8, 392–399 CrossRef CAS
.
- I. Coropceanu and M. G. Bawendi, Nano Lett., 2014, 14, 4097–4101 CrossRef CAS PubMed
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed
.
- A. Pucci, Isr. J. Chem., 2018, 58, 837–844 CrossRef CAS
.
- R. Mori, G. Iasilli, M. Lessi, A. B. Munoz-Garcia, M. Pavone, F. Bellina and A. Pucci, Polym. Chem., 2018, 9, 1168–1177 RSC
.
- D. Nisi, R. Francischello, A. Battisti, A. Panniello, E. Fanizza, M. Striccoli, X. Gu, N. L. C. Leung, B. Z. Tang and A. Pucci, Mater. Chem. Front., 2017, 1, 1406–1412 RSC
.
- F. Gianfaldoni, F. D. Nisi, G. Iasilli, A. Panniello, E. Fanizza, M. Striccoli, D. Ryuse, M. Shimizu, T. Biver and A. Pucci, RSC Adv., 2017, 7, 37302–37309 RSC
.
- J. Yin, D. B. Migas, M. Panahandeh-Fard, S. Chen, Z. Wang, P. Lova and C. Soci, J. Phys. Chem. Lett., 2013, 4, 3303–3309 CrossRef CAS
.
- F. Mateen, H. Oh, W. Jung, M. Binns and S.-K. Hong, Sol. Energy, 2017, 155, 934–941 CrossRef CAS
.
- M. G. Debije, J.-P. Teunissen, M. J. Kastelijn, P. P. C. Verbunt and C. W. M. Bastiaansen, Sol. Energy Mater. Sol. Cells, 2009, 93, 1345–1350 CrossRef CAS
.
- J. C. Goldschmidt, M. Peters, A. Bösch, H. Helmers, F. Dimroth, S. W. Glunz and G. Willeke, Sol. Energy Mater. Sol. Cells, 2009, 93, 176–182 CrossRef CAS
.
- M. Carlotti, G. Ruggeri, F. Bellina and A. Pucci, J. Lumin., 2016, 171, 215–220 CrossRef CAS
.
- P. Minei, E. Fanizza, A. M. Rodriguez, A. B. Munoz-Garcia, P. Cimino, M. Pavone and A. Pucci, RSC Adv., 2016, 6, 17474–17482 RSC
.
- J. C. Goldschmidt and S. Fischer, Adv. Opt. Mater., 2015, 3, 510–535 CrossRef CAS
.
- J. Gutmann, J. Posdziech, M. Peters, L. Steidl, R. Zentel, H. Zappe and J. C. Goldschmidt, Proc. SPIE, 2012, 8438, 843810 CrossRef
.
- A.-L. Joudrier, F. Proise, R. Grapin, J.-L. Pelouard and J.-F. Guillemoles, Energy Procedia, 2014, 60, 173–180 CrossRef CAS
.
- C. Ryan, P. Christian and E. F. Vivian, J. Opt., 2018, 20, 024009 CrossRef
.
- L. Xu, Y. Yao, N. D. Bronstein, L. Li, A. P. Alivisatos and R. G. Nuzzo, ACS Photonics, 2016, 3, 278–285 CrossRef CAS
.
- P. Lova, G. Manfredi, L. Boarino, A. Comite, M. Laus, M. Patrini, F. Marabelli, C. Soci and D. Comoretto, ACS Photonics, 2015, 2, 537–543 CrossRef CAS
.
- P. Lova, C. Bastianini, P. Giusto, M. Patrini, P. Rizzo, G. Guerra, M. Iodice, C. Soci and D. Comoretto, ACS Appl. Mater. Interfaces, 2016, 8, 31941–31950 CrossRef CAS PubMed
.
- P. Lova, V. Grande, G. Manfredi, M. Patrini, S. Herbst, F. Würthner and D. Comoretto, Adv. Opt. Mater., 2017, 5, 1700523 CrossRef
.
- G. Manfredi, P. Lova, F. Di Stasio, R. Krahne and D. Comoretto, ACS Photonics, 2017, 4, 1761–1769 CrossRef CAS
.
- P. Lova, D. Cortecchia, H. N. S. Krishnamoorthy, P. Giusto, C. Bastianini, A. Bruno, D. Comoretto and C. Soci, ACS Photonics, 2018, 5, 867–874 CrossRef CAS
.
- G. Manfredi, P. Lova, F. D. Stasio, P. Rastogi, R. Krahne and D. Comoretto, RSC Adv., 2018, 8, 13026 RSC
.
- P. Giusto, P. Lova, G. Manfredi, S. Gazzo, P. Srinivasan, S. Radice and D. Comoretto, ACS Omega, 2018, 3, 7517–7522 CrossRef CAS
.
- P. Lova, G. Manfredi and D. Comoretto, Adv. Opt. Mater., 2018, 6, 1800730 CrossRef
.
- P. Lova, Polymers, 2018, 10, 1161 CrossRef
.
- M. Shimizu, K. Mochida, M. Katoh and T. Hiyama, J. Phys. Chem. C, 2010, 114, 10004–10014 CrossRef CAS
.
- M. Russo, M. Campoy-Quiles, P. Lacharmoise, T. A. M. Ferenczi, M. Garriga, W. R. Caseri and N. Stingelin, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 65–74 CrossRef CAS
.
- Arduino, https://www.arduino.cc, accessed 10/10/2018.
- WVASE32® software; b. J. A. Woollam Co., Inc.
-
Organic and Hybrid Photonic Crystals, ed. D. Comoretto, Springer International Publishing, Cham, 2015 Search PubMed
.
- T. Kazmierczak, H. Song, A. Hiltner and E. Baer, Macromol. Rapid Commun., 2007, 28, 2210–2216 CrossRef CAS
.
- H. Song, K. Singer, J. Lott, Y. Wu, J. Zhou, J. Andrews, E. Baer, A. Hiltner and C. Weder, J. Mater. Chem., 2009, 19, 7520–7524 RSC
.
- Chamleonlab, https://www.chameleonlab.nl/, http://chameleonlab.nl/, accessed 14/10/2018.
- S. Gazzo, G. Manfredi, R. Poetzsch, Q. Wei, M. Alloisio, B. Voit and D. Comoretto, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 73–80 CrossRef CAS
.
- S. V. Radice, P. Srinivasan, D. Comoretto and S. Gazzo, One-dimensional planar photonic crystals including fluoropolymer compositions and corresponding fabrication methods, WO 2016/087439 (A1), 2016 Search PubMed.
- T. S. Kleine, L. R. Diaz, K. M. Konopka, L. E. Anderson, N. G. Pavlopolous, N. P. Lyons, E. T. Kim, Y. Kim, R. S. Glass, K. Char, R. A. Norwood and J. Pyun, ACS Macro Lett., 2018, 7, 875–880 CrossRef CAS
.
- W. Gaëtan, F. Rolando, S. Stefan and Z. Libero, Macromol. Chem. Phys., 2010, 295, 628–636 Search PubMed
.
- J. Q. Xi, M. F. Schubert, J. K. Kim, E. F. Schubert, M. Chen, S.-Y. Lin, W. Liu and J. A. Smart, Nat. Photonics, 2007, 1, 176–179 CrossRef CAS
.
- L. Frezza, M. Patrini, M. Liscidini and D. Comoretto, J. Phys. Chem. C, 2011, 115, 19939–19946 CrossRef CAS
.
- L. Fornasari, F. Floris, M. Patrini, D. Comoretto and F. Marabelli, Phys. Chem. Chem. Phys., 2016, 18, 14086–14093 RSC
.
- L. Moroni, P. R. Salvi, C. Gellini, G. Dellepiane, D. Comoretto and C. Cuniberti, J. Phys. Chem. A, 2001, 105, 7759–7764 CrossRef CAS
.
- D. Comoretto, C. Cuniberti, G. F. Musso, G. Dellepiane, F. Speroni, C. Botta and S. Luzzati, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 8059–8066 CrossRef CAS
.
- R. J. Gher and R. W. Boyd, Chem. Mater., 1996, 8, 1807–1819 CrossRef
.
- J.-g. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC
.
- M. Russo, S. E. J. Rigby, W. Caseri and N. Stingelin, J. Mater. Chem., 2010, 20, 1348–1356 RSC
.
- T. Yovcheva, I. Vlaeva, I. Bodurov, V. Dragostinova and S. Sainov, Appl. Opt., 2012, 51, 7771–7775 CrossRef CAS PubMed
.
- S. Mahendia, A. Kumar Tomar, P. K. Goyal and S. Kumar, J. Appl. Phys., 2013, 113, 073103 CrossRef
.
- G. Manfredi, C. Mayrhofer, G. Kothleitner, R. Schennach and D. Comoretto, Cellulose, 2016, 23, 2853–2862 CrossRef CAS
.
- Z. Krumer, W. G. J. H. M. van Sark, R. E. I. Schropp and C. de Mello Donegá, Sol. Energy Mater. Sol. Cells, 2017, 167, 133–139 CrossRef CAS
.
- Y. Zhao and R. R. Lunt, Adv. Energy Mater., 2013, 3, 1143–1148 CrossRef CAS
.
- TORAY, https://www.toray.com/, http://www.toray.com, accessed 14/10/2018.
Footnote |
| † Electronic supplementary information (ESI) available: Hybrid titania–PVA nanocomposite optical constants (S1); diffuser and LSC characterization (S2); optical characterization of the DBRs (S3); and optical efficiency measurement details (S4). See DOI: 10.1039/c8qm00595h |
| This journal is © the Partner Organisations 2019 |