β-Cyclodextrin based pH and thermo-responsive biopolymeric hydrogel as a dual drug carrier†
Abstract
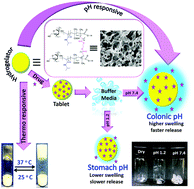
Herein, a novel biocompatible and stimuli-responsive network gel has been developed by grafting and crosslinking poly(N-isopropyl acrylamide) and poly(methacrylic acid) on cyclic oligosaccharide β-cyclodextrin [β-CD-cl-(PNIPAm-co-PMAc)]. Various characterizations (such as NMR spectroscopy, CHN analysis, LCST measurement, and FESEM analysis) have been performed to confirm the formation of copolymer. Swelling characteristics reveal that the developed hydrogel demonstrates dual responsive behaviour (pH and thermo-responsive). The copolymer shows viscoelastic properties and is characterized with excellent yield stress as well as compressive stress. The network hydrogel displays a non-cytotoxic nature towards MG-63 cell lines. The simultaneous in vitro release of metronidazole and ofloxacin from the gel endorses that it released both the colonic drugs simultaneously at the preferred pH (colonic pH 7.4) and body temperature (37 °C) at a desired rate. Finally, the β-CD-cl-(PNIPAm-co-PMAc) gel is probable to be promising for targeted and sustained release of metronidazole and ofloxacin as is obvious from in vivo analysis.


 Please wait while we load your content...
Please wait while we load your content...