A new cluster-based chalcogenide zeolite analogue with a large inter-cluster bridging angle†
Abstract
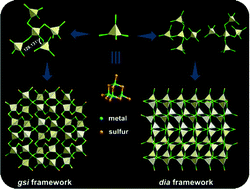
Chalcogenide-based semiconductor zeolite analogues with unique structures have been intensively applied in the fields of gas adsorption, ion exchange and catalysis. However, there are still great limitations in expanding the topological structures of such materials, compared to typical oxide-based zeolites. In this work, a new chalcogenide-based semiconductor zeolite analogue (denoted as SOF-20) with a supertetrahedral T2-InSnS cluster as a secondary building unit (SBU) is reported. Notably, T2 clusters were found to assemble into a zeolitic-like open framework with a gsi topology via an unusually large inter-cluster bridging angle, which is for the first time observed in the family of chalcogenide-based zeolite analogues. Besides, a T2 cluster-based twisted dia framework (denoted as SOF-21) was also created. Both zeolite analogues exhibit good activity in electrocatalytic oxygen reduction reactions (ORRs) due to active Sn components in these semiconducting frameworks.
- This article is part of the themed collection: 2019 Inorganic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...