Potential of N-heterocyclic carbene derivatives from Au13(dppe)5Cl2 gold superatoms. Evaluation of electronic, optical and chiroptical properties from relativistic DFT†‡
Alvaro
Muñoz-Castro

Laboratorio de Química Inorgánica y Materiales Moleculares, Facultad de Ingeniería, Universidad Autonoma de Chile, El Llano Subercaseaux 2801, Santiago, Chile. E-mail: alvaro.munoz@uautonoma.cl
First published on 8th July 2019
Abstract
Atomically precise gold superatoms offer useful templates to evaluate tunable properties via ligand engineering. Herein, the role of different linked N-heterocyclic carbene (NHC) protecting ligands ranging from strong to weak σ-donors was evaluated according to the Tolman electronic parameter (TEP), in species related to the classical [Au13Cl2(dppe)5]3+ nanocluster (1). Our results show a strong dependency on the nature of NHC, providing a useful design principle for the efficient tuning of the structural, optical, chiroptical and emission properties of the Au13Cl2 core. A sizable decrease is observed in the HOMO–LUMO gap for weaker σ-donor ligand cases, with a change in the LUMO nature from core-based orbitals in 1, to a π*-ligand nature. Furthermore, a shorter bridge results in interesting structural changes between the eclipsed ↔ staggered Au13Cl2 core unraveling the potential to convert light energy into mechanical work. Thus, the noticeable modulation of [Au13Cl2(NHC)5]3+ properties by different ligands underlies design rules for tunable clusters towards nanostructured materials, by taking advantage of the recent introduction of NHC-protected gold clusters.
Introduction
The last decade has seen a major advancement in ligand-protected gold nanoclusters owing to their novel optical, electronic and structural features resulting from their unique size-dependent molecule-like properties.1–8 Their physical and chemical properties make them suitable nanomaterials for a wide range of potential applications in catalysis,9,10 biomedicine,11–18 and nanoelectronics,19–21 among others.4,22 These aggregates exhibit rich structural diversity featuring a specific electronic shell structure,23–28 providing the ability to achieve tailorable properties for further applications.10,11,18,29–35,36–40 In pursuit of such development, numerous experimental and theoretical research studies are currently underway towards the rationalization of their structure and stability.41,42,43–50,51–54 Such structures stand on an inner metallic core passivated by ligands,33,55–58 where the electronic and structural characteristics of the core explain the stability of the overall cluster.59–62The 8-ve closed-shell Au13 icosahedron is one of the most recurring motifs, as observed in the earlier [Au13Cl2(dppe)5]3+ cluster characterized by Mingos and coworkers,63 and in the last decade for [Au25(SR)18]−.40,64–66 [Au13Cl2(dppe)5]3+ (1) exhibits interesting optical, luminescence and chiroptical properties as accounted for in separate reports by Konishi and Li research groups,67,68 in comparison with Au9, Au11Cl2 and Au55Cl6 phosphine-protected clusters,69–71 owing to the use of the 1,2-bis(diphenylphosphino)ethane (dppe) ligand as the protecting ligand. 1 possesses singlet-oxygen (1O2) photogeneration capabilities from its lower triplet state (T1), with higher quantum yields than organic dyes and related clusters useful for polymer science to biomedical applications.72,73
The recent introduction of N-heterocyclic carbenes (NHC) as protecting ligands in [C(AuNHC)6]2+ and [Au13(NHC)9Cl3]2+ clusters by Shionoya and Crudden groups,74,75 and explored for Au13Cl2 and Au25Cl2 cores,76 precludes further development of more versatile ligands owing to the NHC characteristics ranging from strong to weak donor ligands according to the Tolman electronic parameter (TEP).77–81 Hence, the incorporation of electron-donor or -withdrawing NHC ligands may strongly influence the molecular properties by tailoring the electronic structure via ligand engineering efforts.82–91
Such features trigger further evaluation and modification of the ligand decorated clusters, by introducing NHC ligand counterparts of the parent dppe ligand in 1, offering a modification of its structural, chiroptical, luminescence and excited state properties. Herein, we explore a proposed series of 8-ve Au13Cl2 cluster-core derivatives involving linked NHC moieties related to dppe, ranging from strong to weak σ-donor ligands, resulting in a chiral arrangement of the ligand-protecting shell related to dppe, which enables the evaluation of their electronic, optical and chiroptical properties. In addition, excited state properties were obtained in order to account for their luminescence behavior in relation to the characterized T1 → S0 emission for 1,68 to explore the tenability owing to the nature of bridged NHC derivatives.
Computational details
All calculations were carried out at the relativistic density functional theory level of theory92 by using the ADF code,93 incorporating scalar corrections via the ZORA Hamiltonian.94 We employed the triple-ξ Slater basis set, plus two polarization functions (STO-TZ2P) for valence electrons, within the generalized gradient approximation (GGA) according to the Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional95,96 because of its reliable performance at reasonable computational cost for similar clusters, allowing a direct comparison with other computational studies of medium-sized gold nanoclusters.97–101The use of the PBE-GGA functional provides accurate results for both structural and optical properties of gold nanoclusters allowing the use of realistic ligands or minimal ligand simplifications,60–65 as denoted by Muniz-Miranda and coworkers,101 when comparing performance between GGA and hybrid functionals. For comparison the calculated HOMO–LUMO gap for [Au13Cl2(dppe)5]3+ amounts to 1.876 eV at the GGA-PBE level, which agrees with the experimentally determined gap of 1.9 eV.67 The gap obtained at the hybrid-PBE0 level amounts to 3.235 eV (ESI, Table S1‡).
The frozen core approximation was applied to the [1s2–4f14] shells for Au, [1s2] for C, and [2s2] for P and Cl, leaving the remaining electrons to be treated variationally. Geometry optimizations of both ground and related excited states were performed without any symmetry restraint, via the analytical energy gradient method implemented by Versluis and Ziegler.102 An energy convergence criterion of 10−4 hartree, gradient convergence criteria of 10−3 hartree per Å and radial convergence criteria of 10−2 Å were employed for the evaluation of the relaxed structures. Electronic excitation energies were calculated via TD-DFT considering the van Leeuwen–Baerends (LB94) xc-functional as in previous similar studies,101,103,104 which offers more accurate values because of its correct asymptotic behavior.105 The current approach has been tested on the description of dual photoluminescence properties of a ligand-supported hexanuclear Au(I) framework, with good agreement with experimental emission and absorption energies.106 Solvent and counterion effects were considered by using the conductor-like screening model107,108 (COSMO) with acetonitrile as the solvent.
Results and discussion
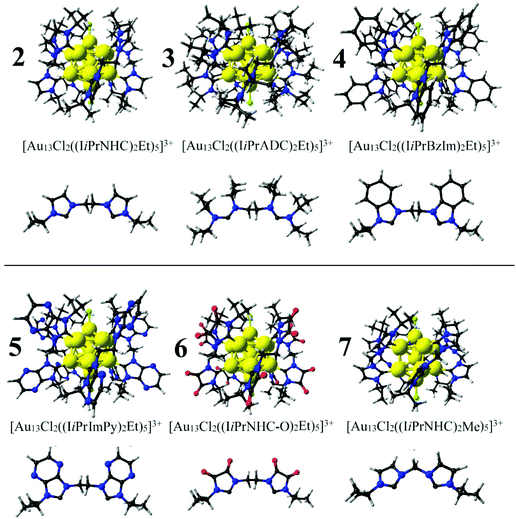
Selected bond lengths for [Au13Cl2(dppe)5]3+ (1) and a related 1,2-bis(N-isopropylimidazolidene) ethane derivative, namely, [Au13Cl2((IiPrNHC)2Et)5]3+ (2), are given in Table 1. The resulting relaxed structures (Fig. 1) exhibit typical Au–Au distances related to ligand-protected gold clusters with an average distance between Au(staple)–Au(center) of 2.622 Å for 1, which compares well with the experimental data available, 2.552 Å,67 with an increase to 2.697 Å when 1,2-bis(N-isopropylimidazolidene)ethane is introduced as a ligand, as calculated for 2. The calculated Au(Cl)–Au(Cl) distance increases from 5.096 to 5.299 Å, denoting an expansion of the icosahedral cage, and similarly, the Au–Cl distances vary from 2.201 to 2.249 Å, respectively. | ||
| Fig. 1 Side (left) and top (right) views of [Au13Cl2(dppe)5]3+ (1) and [Au13Cl2((IiPrNHC)2Et)5]3+ (2). Color code: Au, yellow; Cl, green; P, orange; N, blue; C, black; H, grey. | ||
| 1 | 1 exp.a | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| a Experimental values from ref. 67. | ||||||||
| Au–Au | 2.622 | 2.552 | 2.697 | 2.742 | 2.704 | 2.700 | 2.696 | 2.729 |
| Au–Au(L) | 2.637 | 2.559 | 2.706 | 2.771 | 2.715 | 2.710 | 2.705 | 2.740 |
| Au–Au(Cl) | 2.548 | 2.486 | 2.650 | 2.597 | 2.649 | 2.650 | 2.654 | 2.675 |
| Au(Cl)–Au(Cl) | 5.096 | 4.974 | 5.299 | 5.195 | 5.298 | 5.300 | 5.309 | 5.351 |
| Au–L | 2.185 | 2.071 | 1.961 | 2.075 | 1.966 | 1.958 | 1.950 | 1.964 |
| Au–Cl | 2.201 | 2.169 | 2.249 | 2.267 | 2.246 | 2.237 | 2.217 | 2.246 |
The possibility to achieve carbene ligands with characteristics ranging from strong to weak σ-donor capabilities with different π-acceptor strengths is an advantageous approach to modulate the ligand-protecting layer and tailoring molecular properties. The different σ-donor and π-acceptor character of NHC ligands can be evaluated experimentally by the Tolman electronic parameter (TEP),77 which denotes acyclic diaminocarbene derivatives (ADC, cluster 3, Fig. 2) in the region of strong σ-donor ligands (TEP = 2037 cm−1) with similar steric hindrance as in cluster 2 (TEP = 2050 cm−1). The benzoimidazole derivative (1,2-bis(N-isopropylbenzoimidazolidene) ethane) leads to cluster 4, located in the weak donor region (TEP = 2054 cm−1) with increased π-acceptor capabilities which can account for such NHC-ligand characteristics,109 with a related imidazopyrazine-derivative (ImPy) resulting in cluster 5. Lastly, the oxalamide-based NHC ligand (NHC-O, for short) is included to account for a weaker donor character in cluster 6, with a TEP value of 2069 cm−1, resulting in a higher π-acceptor character,77,109 above 4 and 5. Such ligands lead to the series of ligand-protected clusters, with ligand-donor capabilities in the following order 3 > 4 > 5 > 6, with isopropyl groups as terminal side arms (attached to N). 2 is an intermediate situation between 3 and 4.
Structurally, from 3 to 6, the average Au(staple)–Au(center) distance decreases from 2.742 to 2.696 Å, where the Au(center)–Au(NHC) distances decrease and Au(center)–Au(Cl) increases, leading to an increase of the Au(Cl)–Au(Cl) separation. The Au–NHC distances decrease from 2.075 to 1.950 Å from strong to weak donor ligand capabilities. In addition, the effect of shortening the bridge from two to one carbon atom is evaluated by introducing 1,2-bis(N-isopropylimidazolidene) methane, leading to cluster 7 which exhibits a ∼D5h Au13Cl2 core (eclipsed) in contrast to the related cluster 2 with slightly larger averaged Au(staple)–Au(center) and Au(Cl)–Au(Cl) separations, retaining similar Au-NHC distances.
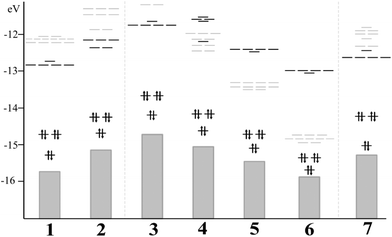
Such species can be formally viewed as 8-ve clusters, denoting a 1S21P6 electronic structure according to the superatom model, where the 1P6 shell remains as the highest-occupied orbital (HOMO) split into two subshells (1Pz21Px,y4) owing to their symmetry (Fig. 3).33,110,111 For the parent [Au13Cl2(dppe)5]3+ (1) cluster, the first low-lying unoccupied levels (LUMO) account for the 1D shell followed by ligand centered orbitals of a π* character. Such features remain similar for cluster 2 (ESI, Fig. S1‡), denoting a related situation between phosphine and NHC-based ligands with a HOMO–LUMO gap of 1.876 and 1.901 eV, respectively, which is also observed for 3 with a gap of 1.909 eV. The calculated HOMO–LUMO gap for the parent cluster 1 is in agreement to the reports by Li and coworkers, denoting an experimental HOMO–LUMO gap of 1.9 eV obtained from ultraviolet photoemission spectroscopy and the measured optical gap.67 Next, 7 exhibits a similar electronic structure to 2, denoting a smaller gap of 1.540 eV. Interestingly, for ligands with weak σ-donor and strong π-acceptor capabilities from 4 to 6, the π*-ligand centered levels are located between the frontier 1P and 1D shells resulting in noteworthy HOMO–LUMO gap decrease from 1.731, 1.201 and 0.380 eV, respectively. Thus, the small frontier orbital gap induced along the series can be an attractive target for further physical studies of unusual optoelectronic properties and multielectron-transfer phenomena.112,113
Optical properties were evaluated to unravel characteristic patterns for different NHC-protected species, reflecting the versatility114,115 introduced by different ligands in the protecting layer.51,60,116–118 For [Au13Cl2(dppe)5]3+ (1), the characterized UV/vis spectra exhibit a peak at 488 nm with a moderate shoulder between 620 to 520 nm, and a larger peak at 383 nm.67 The calculated spectrum (Fig. 4) exhibits three peaks at 590, 473 and 375 nm, in which the first two give rise to the peak with a moderate shoulder with a maximum at 488 nm, and the latter accounts for the larger peak at 383 nm, denoting a good agreement with the experiment supporting the current approach.67 The first peak is given by 1Px,y → π*-ligand manifold transitions, followed by 1Pz → 1D transitions for the second peak, and 5d-Au → 1D transitions for the third peak. For the related [Au13Cl2((IiPrNHC)2Et)5]3+ (2) counterpart, the first peak is calculated at 625 nm denoting a red-shift in comparison with 1, owing to the stabilization of the π*-ligand manifold for NHC derivatives, while the second and third peaks are blue-shifted, at 469 and 336 nm, respectively. Thus, 2 retains similar optical absorption properties to its parent cluster 1.
For stronger σ-donor species (cluster 3), the calculated optical spectrum exhibits a similar shape to 1 and 2, showing the first peak at 576 nm with subsequent peaks at 472 and 373 nm, where for the weaker σ-donor species (4 to 6), a noteworthy red-shift is found owing to the marked decrease in the 1P–π*-ligand gap. For 4, four peaks are present at 690, 614, 517 and 380 nm, the first two peaks are related to 1Px,y → π*-ligand transitions, while the third peak is related to 1Pz → 1D transitions. In 5, the 1Px,y → π*-ligand transitions are now observed as three peaks at 1001, 937 and 876 nm (ESI, Fig. S2‡), followed by the 1Pz → π*-ligand transition at 706 nm, and 1Pz → 1D transitions at 505 and 442 nm, denoting the red-shift of the lower energy peaks. For 6, the observed patterns in the UV/vis spectrum are highly red-shifted according to the lower HOMO–LUMO gap found between 1Px,y and π*-ligand shells (see above), with relevant peaks at 1830 and 1298 nm, besides the related 1Px,y → 1D and 1Pz → 1D transitions at 640, 492 and 423 nm. Far infrared range patterns are given in the ESI.‡
Such features exhibit the strong tuning capabilities of introducing NHC-ligand derivatives along the σ-donor range guided in principle by TEP values, which introduces a noteworthy red-shift for the low-energy peaks of Au13Cl2 core superatoms, owing to their 1P → π*-ligand character. This shows enhanced tunable capabilities in comparison with the related optical properties of thiolate-protected Au25(SR)18 superatoms when different substituents are introduced via SPhX ligands according to the Hammet parameter of –X, denoting that the frontier-orbital gap varies to a small extent, leading to an almost unaffected optical absorption spectrum.118
Furthermore, the related photoluminescence properties of cluster 1,67,68 which provide singlet-oxygen sensitization applications due to the related excited triplet-state (T1)67 are explored for the NHC series. The emission process is related to the T1 → S0 decay67 which is characterized to originate upon excitation from two bands at 490 and 360 nm (as given by its excitation spectrum in acetonitrile)68 of 1P → 1D and 5d-Au → 1D character (Fig. 5). The calculated T1 state exhibits interesting geometrical changes denoted by the increase of the Au(Cl)-Au(Cl) distance from 5.069 to 5.184 Å (Fig. 5 and Table S2‡), leading to a T1–S0 emission calculated at 809 nm (1.533 eV) in line with what was measured experimentally in acetonitrile, 766 nm (1.619 eV), by Konishi and Shichibu.68 Such an emission is solvent dependent owing to the different molecular packing obtained between different solvents, as depicted by the emission at 982 nm (1.263 eV) in dichloromethane.67 This observation shows that molecular aggregation is lower in MeCN than CH2Cl2, which can be further explored as a useful approach to induced emission band variation.
 | ||
| Fig. 5 Variation of Au(Cl)–Au(Cl) at the emissive excited T1 state. Color code: Au, yellow; Cl, green; P, orange; N, blue; C, black; H, grey. | ||
For the related NHC derivative, [Au13Cl2(IiPr)5]3+ (2), the T1-geometry features greater Au(Cl)–Au(Cl) elongation from 5.299 to 5.711 Å (ΔAu(Cl)–Au(Cl) = 0.412 Å), which is larger than that calculated for 1 (ΔAu(Cl)–Au(Cl) = 0.088 Å). The obtained T1 → S0 emission is located at 1015 nm (1.221 eV), which despite the similar ground-state electronic structure and the larger geometrical distortion at T1, leads to a decrease in the 1D–1P gap, resulting in an emission red-shift, as expected from our calculations. For 3, the similar frontier orbital characteristics to 1 result in a similar T1 → S0 emission at 875 nm (1.417 eV) with slight structural modifications at the T1 excited state. Moreover, for the 4–6 series, the calculated emission is related to the decrease of the HOMO–LUMO gap at the ground state (S0), with values of 0.682, 0.686 and 0.342 eV, respectively, which can be interesting far-infrared emitter species to probe experimentally, with similar excitation wavelengths to 1 owing to the similar 1P → 1D transition values.
For 7, the effect of bridge shortening from –CH2CH2– to –CH2– results in a lower HOMO–LUMO gap (1.540 eV) in comparison with 2 (1.901 eV), which leads to a red-shift of the first peaks related to 1P → 1D and 5d-Au → 1D transitions (725 and 645 nm). The T1 → S0 emission is calculated at 0.821 eV (1510 nm) and exhibits a ∼D5h-/∼D5d-Au13Cl2 core transformation, which can be an interesting feature to explore further light-driven molecular motors (ESI, Fig. S3‡).119
Lastly, the circular dichroism (CD) spectrum is evaluated owing to the chiral arrangement of the protecting ligands supporting the achiral Au13Cl2 core, which results from the use of dppe (1), and related bridged NHC derivatives (2–7). All the studied species exhibit optical activity denoting the effective chiral pattern constructed by the protecting units (Fig. 6). The calculated CD for the right-handed enantiomer of 1 shows similar patterns to that observed experimentally for enantiomer 2 by Li and coworkers,67 with a positive peak at 560 nm (exp.: 563 nm), followed by a negative peak at 462 nm (exp.: 472 nm), and a last signal as a positive peak at 345 nm (exp.: 386 nm). For 2, two negative peaks are expected at 578 and 462 nm, before a positive peak at 331 nm, in line with the trend observed for the UV/vis (see above). For the series from 3 to 7, the variation of the CD patterns is related to the red-shift variation observed for the 1P → π*-ligand transitions, where it is particularly modified for 5, 6 and 7, denoting the higher tuning capabilities introduced by metal-to-ligand centered transitions.
 | ||
| Fig. 6 Calculated circular dichroism spectra or right-handed isomers for 1–7. Gaussian broadening of 0.6 eV was employed for 1 and 2, to match the experimentally observed CD for 1 (background grey line), a broadening of 0.2 eV was employed in other cases. Values between ±500 × 10−40 esu2 cm2. Lower energy peaks for 5 and 6 are shown in ESI Fig. S4.‡ | ||
Conclusions
In summary, the role of different NHC-linked protecting ligands ranging from strong to weak σ-donors according to TPE values was evaluated on the structural, optical, chiroptical and emission properties of the Au13Cl2 core, derived from the [Au13Cl2(dppe)5]3+ nanocluster. Our findings provide a useful design principle for efficient tuning by selected NHC ligands resulting in a noteworthy variation of electronic properties, and thus, modification of related cluster characteristics. A comparison between [Au13Cl2(dppe)5]3+ and [Au13Cl2((IiPrNHC)2Et)5]3+ reveals similar characteristics of related 8-ve superatoms, denoting a red-shift for the lower energy peaks in the optical absorption spectrum ascribed to 1Px,y → π*-ligand manifold transitions, owing to the stabilization of the π*-ligand levels for the later. Also, a blue-shift is observed for 1Pz → 1D and 5d-Au → 1D transitions. The calculated emission peaks denote a red-shift from 809 to 1015 nm, respectively, owing to a larger distortion at the emissive excited state for the later. From 3 to 6, it is shown that weaker ligand-donor capabilities significantly decrease the frontier orbital gap with a change in the 1P–1D to a 1P–π*-ligand character. Lastly, the effect of shortening the bridge between linked NHC in [Au13Cl2((IiPrNHC)2Me)5]3+ results in a ∼D5h eclipsed Au13Cl2 core, in contrast to [Au13Cl2((IiPrNHC)2Et)5]3+, denoting interesting structural changes in the eclipsed ↔ staggered Au13Cl2 core unraveling the potential to convert light energy into mechanical work.Such a ligand-tailored behavior of [Au13Cl2(NHC)5]3+ derivatives appears as an addition to the useful strategies to reduce HOMO–LUMO separation, which underlies design rules towards novel clusters for building blocks of nanostructured materials, for optical, chiroptical and structural modification tuning towards further applications of superatomic clusters.
Author contributions
The manuscript was written through the contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by FONDECYT 1180683.References
- C. N. R. Rao, A. Müller and A. K. Cheetham, in The Chemistry of Nanomaterials, ed. C. N. R. Rao, A. Müller and A. K. Cheetham, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG, 2004. DOI:10.1002/352760247X.
- Y. Sun, Shape-Controlled Synthesis of Gold and Silver Nanoparticles, Science, 2002, 298, 2176–2179, DOI:10.1126/science.1077229.
- Y. Yin and D. Talapin, The Chemistry of Functional Nanomaterials, Chem. Soc. Rev., 2013, 42, 2484, 10.1039/c3cs90011h.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities, Chem. Rev., 2016, 116, 10346–10413, DOI:10.1021/acs.chemrev.5b00703.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, Precise Synthesis, Functionalization and Application of Thiolate-Protected Gold Clusters, Coord. Chem. Rev., 2016, 320–321, 238–250, DOI:10.1016/j.ccr.2016.02.013.
- A. Fernando, K. L. D. M. Weerawardene, N. V. Karimova and C. M. Aikens, Quantum Mechanical Studies of Large Metal, Metal Oxide, and Metal Chalcogenide Nanoparticles and Clusters, Chem. Rev., 2015, 115, 6112–6216, DOI:10.1021/cr500506r.
- T. Stoll, E. Sgrò, J. W. Jarrett, J. Réhault, A. Oriana, L. Sala, F. Branchi, G. Cerullo and K. L. Knappenberger, Superatom State-Resolved Dynamics of the Au25(SC8H9)18- Cluster from Two-Dimensional Electronic Spectroscopy, J. Am. Chem. Soc., 2016, 138, 1788–1791, DOI:10.1021/jacs.5b12621.
- R. Costi, A. E. Saunders and U. Banin, Colloidal Hybrid Nanostructures: A New Type of Functional Materials, Angew. Chem., Int. Ed., 2010, 49, 4878–4897, DOI:10.1002/anie.200906010.
- I. Cano, A. M. Chapman, A. Urakawa and P. W. N. M. van Leeuwen, Air-Stable Gold Nanoparticles Ligated by Secondary Phosphine Oxides for the Chemoselective Hydrogenation of Aldehydes: Crucial Role of the Ligand, J. Am. Chem. Soc., 2014, 136, 2520–2528, DOI:10.1021/ja411202h.
- M.-C. Daniel and D. Astruc, Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology, Chem. Rev., 2004, 104, 293–346, DOI:10.1021/cr030698+.
- Y. Levi-Kalisman, P. D. Jadzinsky, N. Kalisman, H. Tsunoyama, T. Tsukuda, D. A. Bushnell and R. D. Kornberg, Synthesis and Characterization of Au102 (p-MBA) 44 Nanoparticles, J. Am. Chem. Soc., 2011, 133, 2976–2982 CrossRef CAS PubMed.
- T. Tsukuda, H. Tsunoyama and H. Sakurai, Aerobic Oxidations Catalyzed by Colloidal Nanogold, Chem. – Asian J., 2011, 6, 736–748, DOI:10.1002/asia.201000611.
- Y. Zhu, H. Qian and R. Jin, Catalysis Opportunities of Atomically Precise Gold Nanoclusters, J. Mater. Chem., 2011, 21, 6793–6799, 10.1039/c1jm10082c.
- K. Kwak, S. S. Kumar and D. Lee, Selective Determination of Dopamine Using Quantum-Sized Gold Nanoparticles Protected with Charge Selective Ligands, Nanoscale, 2012, 4, 4240–4246, 10.1039/c2nr30481c.
- N. Sakai and T. Tatsuma, Photovoltaic Properties of Glutathione-Protected Gold Clusters Adsorbed on TiO2 Electrodes, Adv. Mater., 2010, 22, 3185–3188, DOI:10.1002/adma.200904317.
- Z. Wu, M. Wang, J. Yang, X. Zheng, W. Cai, G. Meng, H. Qian, H. Wang and R. Jin, Well-Defined Nanoclusters as Fluorescent Nanosensors: A Case Study on Au25(SG)18, Small, 2012, 8, 2028–2035, DOI:10.1002/smll.201102590.
- R. W. Murray, Nanoelectrochemistry: Metal Nanoparticles, Nanoelectrodes, and Nanopores, Chem. Rev., 2008, 108, 2688–2720, DOI:10.1021/cr068077e.
- J. M. Galloway, J. P. Bramble, A. E. Rawlings, G. Burnell, S. D. Evans and S. S. Staniland, Biotemplated Magnetic Nanoparticle Arrays, Small, 2012, 8, 204–208, DOI:10.1002/smll.201101627.
- Z. Wu and R. Jin, On the Ligand's Role in the Fluorescence of Gold Nanoclusters, Nano Lett., 2010, 10, 2568–2573, DOI:10.1021/nl101225f.
- L. Li, H. Liu, Y. Shen, J. Zhang and J.-J. Zhu, Electrogenerated Chemiluminescence of Au Nanoclusters for the Detection of Dopamine, Anal. Chem., 2011, 83, 661–665, DOI:10.1021/ac102623r.
- M. De Nardi, S. Antonello, D. Jiang, F. Pan, K. Rissanen, M. Ruzzi, A. Venzo, A. Zoleo and F. Maran, Gold Nanowired: A Linear (Au 25) n Polymer from Au 25 Molecular Clusters, ACS Nano, 2014, 8, 8505–8512, DOI:10.1021/nn5031143.
- Z. Luo, A. W. Castleman and S. N. Khanna, Reactivity of Metal Clusters, Chem. Rev., 2016, 116, 14456–14492, DOI:10.1021/acs.chemrev.6b00230.
- R. Jin, Atomically Precise Metal Nanoclusters: Stable Sizes and Optical Properties, Nanoscale, 2015, 7, 1549–1565, 10.1039/C4NR05794E.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, Correlating the Crystal Structure of a Thiol-Protected Au25 Cluster and Optical Properties, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- P. M. Shem, R. Sardar and J. S. Shumaker-Parry, One-Step Synthesis of Phosphine-Stabilized Gold Nanoparticles Using the Mild Reducing Agent 9-BBN, Langmuir, 2009, 25, 13279–13283, DOI:10.1021/la903003n.
- T. G. M. M. Kappen, J. J. Bour, P. P. J. Schlebos, A. M. Roelofsen, J. G. M. Van der Linden, J. J. Steggerda, M. A. Aubart, D. A. Krogstad, M. F. J. Schoondergang and L. H. Pignolet, Reaction of Platinum-Gold Cluster Compounds with Dihydrogen, Inorg. Chem., 1993, 32, 1074–1075, DOI:10.1021/ic00059a007.
- L. H. Pignolet, M. A. Aubart, K. L. Craighead, R. A. T. Gould, D. A. Krogstad and J. S. Wiley, Phosphine-Stabilized, Platinum-Gold and Palladium-Gold Cluster Compounds and Applications in Catalysis, Coord. Chem. Rev., 1995, 143, 219–263, DOI:10.1016/0010-8545(94)07009-9.
- R. C. B. Copley, C. M. Hill and D. M. P. Mingos, Synthesis and Structural Characterization of [Au2Pd14(μ3-CO)7(μ2-CO)2(PMe3)11](PF6)2An Icosahedrally-Based High Nuclearity Mixed Metal Cluster, J. Cluster Sci., 1995, 6, 71–91, DOI:10.1007/BF01175837.
- H. Qian, Y. Zhu and R. Jin, Size-Focusing Synthesis, Optical and Electrochemical Properties of Monodisperse Au38(SC2H4Ph)24 Nanoclusters, ACS Nano, 2009, 3, 3795–3803 CrossRef CAS PubMed.
- R. L. Whetten, J. T. Khoury, M. M. Alvarez, S. Murthy, I. Vezmar, Z. L. Wang, P. W. Stephens, C. L. Cleveland, W. D. Luedtke and U. Landman, Nanocrystal Gold Molecules, Adv. Mater., 1996, 8, 428–433 CrossRef CAS.
- L. A. Oro, P. Braunstein and P. R. Raithby, Metal Clusters in Chemistry, Wiley-vch Weinheim, Germany, 1999, vol. 3 Search PubMed.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, Crystal Structure of the Gold Nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18], J. Am. Chem. Soc., 2008, 130, 3754–3755, DOI:10.1021/ja800561b.
- M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck, H. Häkkinen and H. Gronbeck, et al., A Unified View of Ligand-Protected Gold Clusters as Superatom Complexes, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9157–9162, DOI:10.1073/pnas.0801001105.
- P. Jena, Beyond the Periodic Table of Elements: The Role of Superatoms, J. Phys. Chem. Lett., 2013, 4, 1432–1442, DOI:10.1021/jz400156t.
- S. Knoppe, I. Dolamic and T. Bürgi, Racemization of a Chiral Nanoparticle Evidences the Flexibility of the Gold-Thiolate Interface, J. Am. Chem. Soc., 2012, 134, 13114–13120, DOI:10.1021/ja3053865.
- A. C. Reber and S. N. Khanna, Superatoms: Electronic and Geometric Effects on Reactivity, Acc. Chem. Res., 2017, 50, 255–263, DOI:10.1021/acs.accounts.6b00464.
- C. Zeng, Y. Chen, A. Das and R. Jin, Transformation Chemistry of Gold Nanoclusters: From One Stable Size to Another, J. Phys. Chem. Lett., 2015, 61, 2976–2986, DOI:10.1021/acs.jpclett.5b01150.
- Y. Gao, S. Bulusu and X. C. Zeng, A Global Search of Highly Stable Gold-Covered Bimetallic Clusters M@Aun (n=8–17): Endohedral Gold Clusters, ChemPhysChem, 2006, 7, 2275–2278, DOI:10.1002/cphc.200600472.
- A. M. S. Pembere, C. Cui, H. Wu and Z. Luo, Small Gold Clusters Catalyzing Oxidant-Free Dehydrogenation of Glycerol Initiated by Methene Hydrogen Atom Transfer, Chin. Chem. Lett., 2019, 30, 1000–1004, DOI:10.1016/j.cclet.2018.12.019.
- W. Ding, C. Huang, L. Guan, X. Liu, Z. Luo and W. Li, Water-Soluble Au13 Clusters Protected by Binary Thiolates: Structural Accommodation and the Use for Chemosensing, Chem. Phys. Lett., 2017, 676, 18–24, DOI:10.1016/j.cplett.2017.03.036.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Toward Total Synthesis of Thiolate-Protected Metal Nanoclusters, Acc. Chem. Res., 2018, 51, 1338–1348, DOI:10.1021/acs.accounts.8b00065.
- N. A. Sakthivel and A. Dass, Aromatic Thiolate-Protected Series of Gold Nanomolecules and a Contrary Structural Trend in Size Evolution, Acc. Chem. Res., 2018, 51, 1774–1783, DOI:10.1021/acs.accounts.8b00150.
- Q.-F. Zhang, X. Chen and L.-S. Wang, Toward Solution Syntheses of the Tetrahedral Au20 Pyramid and Atomically Precise Gold Nanoclusters with Uncoordinated Sites, Acc. Chem. Res., 2018, 51, 2159–2168, DOI:10.1021/acs.accounts.8b00257.
- A. W. Cook and T. W. Hayton, Case Studies in Nanocluster Synthesis and Characterization: Challenges and Opportunities, Acc. Chem. Res., 2018, 51, 2456–2464, DOI:10.1021/acs.accounts.8b00329.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Alkynyl Approach toward the Protection of Metal Nanoclusters, Acc. Chem. Res., 2018, 51, 2465–2474, DOI:10.1021/acs.accounts.8b00359.
- S. Sharma, K. K. Chakrahari, J.-Y. Saillard and C. W. Liu, Structurally Precise Dichalcogenolate-Protected Copper and Silver Superatomic Nanoclusters and Their Alloys, Acc. Chem. Res., 2018, 51, 2475–2483, DOI:10.1021/acs.accounts.8b00349.
- T. Higaki, Q. Li, M. Zhou, S. Zhao, Y. Li, S. Li and R. Jin, Toward the Tailoring Chemistry of Metal Nanoclusters for Enhancing Functionalities, Acc. Chem. Res., 2018, 51, 2764–2773, DOI:10.1021/acs.accounts.8b00383.
- Q. Tang, G. Hu, V. Fung and D. Jiang, Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles, Acc. Chem. Res., 2018, 51, 2793–2802, DOI:10.1021/acs.accounts.8b00380.
- K. Konishi, M. Iwasaki and Y. Shichibu, Phosphine-Ligated Gold Clusters with Core+ Exo Geometries: Unique Properties and Interactions at the Ligand–Cluster Interface, Acc. Chem. Res., 2018, 51, 3125–3133, DOI:10.1021/acs.accounts.8b00477.
- J. Yan, B. K. Teo and N. Zheng, Surface Chemistry of Atomically Precise Coinage–Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis, Acc. Chem. Res., 2018, 51, 3084–3093, DOI:10.1021/acs.accounts.8b00371.
- C. M. Aikens, Electronic and Geometric Structure, Optical Properties, and Excited State Behavior in Atomically Precise Thiolate-Stabilized Noble Metal Nanoclusters, Acc. Chem. Res., 2018, 51, 3065–3073, DOI:10.1021/acs.accounts.8b00364.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Atomic-Level Doping of Metal Clusters, Acc. Chem. Res., 2018, 51, 3094–3103, DOI:10.1021/acs.accounts.8b00412.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Alloy Clusters: Precise Synthesis and Mixing Effects, Acc. Chem. Res., 2018, 51, 3114–3124, DOI:10.1021/acs.accounts.8b00453.
- B. Bhattarai, Y. Zaker, A. Atnagulov, B. Yoon, U. Landman and T. P. Bigioni, Chemistry and Structure of Silver Molecular Nanoparticles, Acc. Chem. Res., 2018, 51, 3104–3113, DOI:10.1021/acs.accounts.8b00445.
- A. W. Castleman and S. N. Khanna, Clusters, Superatoms, and Building Blocks of New Materials, J. Phys. Chem. C, 2009, 113, 2664–2675, DOI:10.1021/jp806850h.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 A Resolution, Science, 2007, 318, 430–433, DOI:10.1126/science.1148624.
- S. A. Claridge, A. W. Castleman, S. N. Khanna, C. B. Murray, A. Sen and P. S. Weiss, Cluster-Assembled Materials, ACS Nano, 2009, 3, 244–255, DOI:10.1021/nn800820e.
- T. Bürgi, Properties of the Gold-Sulphur Interface: From Self-Assembled Monolayers to Clusters, Nanoscale, 2015, 7, 15553–15567, 10.1039/C5NR03497C.
- F. Gam, D. Paez-Hernandez, R. Arratia-Perez, C. W. Liu, S. Kahlal, J.-Y. Saillard and A. Muñoz-Castro, Coinage Metal Superatomic Cores: Insights into Their Intrinsic Stability and Optical Properties from Relativistic DFT Calculations, Chem. – Eur. J., 2017, 23, 11330–11337, DOI:10.1002/chem.201701673.
- T. Tsukuda and H. Häkkinen, Protected Metal Clusters: From Fundamentals to Applications, Elsevier, 2015 Search PubMed.
- J.-Y. Saillard and J.-F. Halet, Structure and Bonding Patterns in Large Molecular Ligated Metal Clusters, Struct. Bonding, 2016, 157–179, DOI:10.1007/430_2015_210.
- T. P. Fehlner, J.-F. Halet and J.-Y. Saillard, Molecular Clusters. A Bridge to Solid State Chemistry. Cambridge Univ. Press, Cambridge, Gd., 2007, p. 378 Search PubMed.
- C. E. Briant, B. R. C. Theobald, J. W. White, L. K. Bell, D. M. P. Mingos and A. J. Welch, Synthesis and X-Ray Structural Characterization of the Centred Icosahedral Gold Cluster Compound [Au13(PMe2Ph)10Cl2](PF6)3; the Realization of a Theoretical Prediction, J. Chem. Soc., Chem. Commun., 1981,(5), 201–202, 10.1039/c39810000201.
- F. K. Sheong, J.-X. Zhang and Z. Lin, An [Au13]5+ Approach to the Study of Gold Nanoclusters, Inorg. Chem., 2016, 55, 11348–11353, DOI:10.1021/acs.inorgchem.6b01881.
- M. Laupp and J. Strähle, [(Ph3PAu)6(DppeAu2)(AuCl)4Pd], an Icosahedral Au12 Cluster with a Central Pd Atom, Angew. Chem., Int. Ed. Engl., 1994, 33(2), 207–209, DOI:10.1002/anie.199402071.
- X. Kang, H. Chong and M. Zhu, Au25(SR)18: The Captain of the Great Nanocluster Ship, Nanoscale, 2018, 10, 10758–10834, 10.1039/C8NR02973C.
- J. Zhang, Y. Zhou, K. Zheng, H. Abroshan, D. R. Kauffman, J. Sun and G. Li, Diphosphine-Induced Chiral Propeller Arrangement of Gold Nanoclusters for Singlet Oxygen Photogeneration, Nano Res., 2017, 1–12, DOI:10.1007/s12274-017-1935-2.
- Y. Shichibu and K. Konishi, HCl-Induced Nuclearity Convergence in Diphosphine-Protected Ultrasmall Gold Clusters: A Novel Synthetic Route to “Magic-Number” Au13 Clusters, Small, 2010, 6, 1216–1220, DOI:10.1002/smll.200902398.
- F. Wen, U. Englert, B. Gutrath and U. Simon, Crystal Structure, Electrochemical and Optical Properties of [Au9(PPh3)8](NO3)3, Eur. J. Inorg. Chem., 2008, 1, 106–111, DOI:10.1002/ejic.200700534.
- G. H. Woehrle, M. G. Warner and J. E. Hutchison, Ligand Exchange Reactions Yield Subnanometer, Thiol-Stabilized Gold Particles with Defined Optical Transitions, J. Phys. Chem. B, 2002, 106, 9979–9981, DOI:10.1021/jp025943s.
- G. Schmid, R. Pfeil, R. Boese, F. Bandermann, S. Meyer, G. H. M. Calis and J. W. A. van der Velden, Au55[P(C6H5)3]12Cl6—Ein Goldcluster Ungewöhnlicher Größe, Chem. Ber., 1981, 114, 3634–3642, DOI:10.1002/cber.19811141116.
- D. Ashen-Garry and M. Selke, Singlet Oxygen Generation by Cyclometalated Complexes and Applications, Photochem. Photobiol., 2014, 90, 257–274, DOI:10.1111/php.12211.
- M. DeRosa, Photosensitized Singlet Oxygen and Its Applications, Coord. Chem. Rev., 2002, 233, 351–371, DOI:10.1016/S0010-8545(02)00034-6.
- H. Ube, Q. Zhang and M. Shionoya, Organometallics, 2018, 37, 2007–2009, DOI:10.1021/acs.organomet.8b00291.
- M. Narouz, S. Takano, P. Lummis, T. Levchenko, A. Nazemi, S. Kaappa, S. Malola, S. Yousefalizadeh, K. Stamplecoskie, H. Häkkinen, T. Tsukuda and C. Crudden, N-Heterocyclic Carbene-Stabilized Au13 Superatom Clusters, ChemRxiv, 2018 DOI:10.26434/chemrxiv.7498748.v1.
- D. MacLeod Carey and A. Muñoz-Castro, Evaluation of N-Heterocyclic Carbene Counterparts of Classical Gold Clusters; Bonding Properties of Octahedral CAu6, Icosahedral Au13Cl2, and Bi-icosahedral Au25Cl2 Cores from Relativistic DFT Calculations, J. Phys. Chem. C, 2019, 123, 12466–12473, DOI:10.1021/acs.jpcc.9b01254.
- C. A. Tolman, Steric Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous Catalysis, Chem. Rev., 1977, 77, 313–348, DOI:10.1021/cr60307a002.
- D. Munz, Pushing Electrons—Which Carbene Ligand for Which Application?, Organometallics, 2018, 37, 275–289, DOI:10.1021/acs.organomet.7b00720.
- D. G. Gusev and E. Peris, The Tolman Electronic Parameter (TEP) and the Metal–Metal Electronic Communication in Ditopic NHC Complexes, Dalton Trans., 2013, 42, 7359, 10.1039/c3dt32959c.
- A. J. Arduengo and G. Bertrand, Carbenes Introduction., Chem. Rev., 2009, 109, 3209–3210, DOI:10.1021/cr900241h.
- D. J. Nelson and S. P. Nolan, Quantifying and Understanding the Electronic Properties of N-Heterocyclic Carbenes, Chem. Soc. Rev., 2013, 42, 6723, 10.1039/c3cs60146c.
- G. Li, H. Abroshan, C. Liu, S. Zhuo, Z. Li, Y. Xie, H. J. Kim, N. L. Rosi and R. Jin, Tailoring the Electronic and Catalytic Properties of Au 25 Nanoclusters via Ligand Engineering, ACS Nano, 2016, 10, 7998–8005, DOI:10.1021/acsnano.6b03964.
- H. Yang, Y. Wang, J. Lei, L. Shi, X. Wu, V. Mäkinen, S. Lin, Z. Tang, J. He and H. Häkkinen, et al., Ligand-Stabilized Au13Cux(x=2, 4, 8) Bimetallic Nanoclusters: Ligand Engineering to Control the Exposure of Metal Sites, J. Am. Chem. Soc., 2013, 135, 9568–9571, DOI:10.1021/ja402249s.
- X. Yuan, N. Goswami, I. Mathews, Y. Yu and J. Xie, Enhancing Stability through Ligand-Shell Engineering: A Case Study with Au25(SR)18 Nanoclusters, Nano Res., 2015, 8, 3488–3495, DOI:10.1007/s12274-015-0847-2.
- X.-K. Wan, J.-Q. Wang, Z.-A. Nan and Q.-M. Wang, Ligand Effects in Catalysis by Atomically Precise Gold Nanoclusters, Sci. Adv., 2017, 3, e1701823, DOI:10.1126/sciadv.1701823.
- N. V. Karimova and C. M. Aikens, Chiroptical Activity in BINAP- and DIOP-Stabilized Octa- and Undecagold Clusters, J. Phys. Chem. C, 2018, 122, 11051–11065, DOI:10.1021/acs.jpcc.7b12264.
- Y. Yang, H. Xu, D. Cao, X. C. Zeng and D. Cheng, Hydrogen Production via Efficient Formic Acid Decomposition: Engineering the Surface Structure of Pd-Based Alloy Catalysts by Design, ACS Catal., 2019, 9, 781–790, DOI:10.1021/acscatal.8b03485.
- H. Xu, D. Cheng, Y. Gao and X. C. Zeng, Assessment of Catalytic Activities of Gold Nanoclusters with Simple Structure Descriptors, ACS Catal., 2018, 8, 9702–9710, DOI:10.1021/acscatal.8b02423.
- A. A. Danopoulos, T. Simler and P. Braunstein, N-Heterocyclic, Carbene Complexes of Copper, Nickel, and Cobalt, Chem. Rev., 2019, 119, 3730–3961, DOI:10.1021/acs.chemrev.8b00505.
- P. Ai, M. Mauro, C. Gourlaouen, S. Carrara, L. De Cola, Y. Tobon, U. Giovanella, C. Botta, A. A. Danopoulos and P. Braunstein, Bonding, Luminescence, Metallophilicity in Linear Au3 and Au2Ag Chains Stabilized by Rigid Diphosphanyl NHC Ligands, Inorg. Chem., 2016, 55, 8527–8542, DOI:10.1021/acs.inorgchem.6b01095.
- P. Ai, A. A. Danopoulos and P. Braunstein, Aurophilicity-Triggered Assembly of Novel Cyclic Penta- and Hexanuclear Gold(I) Complexes with Rigid Anionic NHC-Type Ligands, Inorg. Chem., 2015, 54, 3722–3724, DOI:10.1021/acs.inorgchem.5b00276.
- K. G. Dyall and K. Fægri, Introduction to Relativistic Quantum Chemistry, Oxford University Press, New York, 2007 Search PubMed.
- Amsterdam Density Functional (ADF) Code, Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com.
- E. van Lenthe, E.-J. J. Baerends and J. G. Snijders, Relativistic Total Energy Using Regular Approximations, J. Chem. Phys., 1994, 101, 9783, DOI:10.1063/1.467943.
- J. P. Perdew, K. Burke and Y. Wang, Generalized Gradient Approximation for the Exchange-Correlation Hole of a Many-Electron System, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 16533–16539, DOI:10.1103/PhysRevB.54.16533.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1997, 78, 1396–1396, DOI:10.1103/PhysRevLett.78.1396.
- O. Lopez-Acevedo, J. Akola, R. L. Whetten, H. Gronbeck and H. Hakkinen, Structure and Bonding in the Ubiquitous Icosahedral Metallic Gold Cluster Au144 (SR) 60, J. Phys. Chem. C, 2009, 113, 5035–5038 CrossRef CAS.
- J. Akola, M. Walter, R. L. Whetten, H. Häkkinen and H. Grönbeck, On the Structure of Thiolate-Protected Au25, J. Am. Chem. Soc., 2008, 130, 3756–3757, DOI:10.1021/ja800594p.
- D. Jiang, The Expanding Universe of Thiolated Gold Nanoclusters and Beyond, Nanoscale, 2013, 5, 7149–7160, 10.1039/c3nr34192e.
- J. Akola, K. A. Kacprzak, O. Lopez-Acevedo, M. Walter, H. Grönbeck and H. Häkkinen, Thiolate-Protected Au25 Superatoms as Building Blocks: Dimers and Crystals, J. Phys. Chem. C, 2010, 114, 15986–15994, DOI:10.1021/jp1015438.
- F. Muniz-Miranda, M. Cristina Menziani and A. Pedone, Assessment of Exchange-Correlation Functionals in Reproducing the Structure and Optical Gap of Organic-Protected Gold Nanoclusters, J. Phys. Chem. C, 2014, 118, 7532–7544, DOI:10.1021/jp411483x.
- L. Versluis and T. Ziegler, The Determination of Molecular Structures by Density Functional Theory. The Evaluation of Analytical Energy Gradients by Numerical Integration, J. Chem. Phys., 1988, 88, 322–328, DOI:10.1063/1.454603.
- O. Lopez-Acevedo, H. Tsunoyama, T. Tsukuda, H. Häkkinen and C. M. Aikens, Chirality and Electronic Structure of the Thiolate-Protected Au38 Nanocluster, J. Am. Chem. Soc., 2010, 132, 8210–8218, DOI:10.1021/ja102934q.
- F. Alkan, A. Muñoz-Castro and C. M. Aikens, Relativistic DFT Investigation of Electronic Structure Effects Arising from Doping the Au25 Nanocluster with Transition Metals, Nanoscale, 2017, 9, 15825–15834, 10.1039/C7NR05214F.
- S. J. A. van Gisbergen, F. Kootstra, P. R. T. Schipper, O. V. Gritsenko, J. G. Snijders and E. J. Baerends, Density-Functional-Theory Response-Property Calculations with Accurate Exchange-Correlation Potentials, Phys. Rev. A, 1998, 57(4), 2556–2571, DOI:10.1103/PhysRevA.57.2556.
- P. Ai, M. Mauro, A. A. Danopoulos, A. Muñoz-Castro and P. Braunstein, Dual Emission of a Cyclic Hexanuclear Gold(I) Complex. Interplay between Au3 and Au2 Ligand-Supported Luminophores, J. Phys. Chem. C, 2019, 123, 915–921, DOI:10.1021/acs.jpcc.8b10190.
- A. Klamt and G. Schüürmann, COSMO: A New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and Its Gradient, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805, 10.1039/P29930000799.
- A. Klamt and V. Jonas, Treatment of the Outlying Charge in Continuum Solvation Models, J. Chem. Phys., 1996, 105, 9972, DOI:10.1063/1.472829.
- K. Verlinden, H. Buhl, W. Frank and C. Ganter, Determining the Ligand Properties of N-Heterocyclic Carbenes from 77Se NMR Parameters, Eur. J. Inorg. Chem., 2015, 2015, 2416–2425, DOI:10.1002/ejic.201500174.
- H. Häkkinen, Electronic Structure: Shell Structure and the Superatom Concept. in Protected Metal Clusters: From Fundamentals to Applications, ed. H. Hakkinen and T. Tsukuda, Elsevier Science, 2015, pp. 189–222. DOI:10.1016/B978-0-08-100086-1.00008-7.
- O. Lopez-Acevedo, J. Akola, R. L. Whetten, H. Grönbeck and H. Häkkinen, Structure and Bonding in the Ubiquitous Icosahedral Metallic Gold Cluster Au144(SR)60., J. Phys. Chem. C, 2009, 113, 5035–5038, DOI:10.1021/jp8115098.
- D. F. Perepichka and M. R. Bryce, Molecules with Exceptionally Small HOMO-LUMO Gaps, Angew. Chem., Int. Ed., 2005, 44, 5370–5373, DOI:10.1002/anie.200500413.
- A. Mailman, A. A. Leitch, W. Yong, E. Steven, S. M. Winter, R. C. M. Claridge, A. Assoud, J. S. Tse, S. Desgreniers and R. A. Secco, et al., The Power of Packing: Metallization of an Organic Semiconductor, J. Am. Chem. Soc., 2017, 139, 2180–2183, DOI:10.1021/jacs.6b12814.
- J. Jung, S. Kang and Y.-K. Han, Ligand Effects on the Stability of Thiol-Stabilized Gold Nanoclusters: Au25(SR)18−, Au38(SR)24, and Au102(SR)44, Nanoscale, 2012, 4, 4206–4210, 10.1039/c2nr30501a.
- R. Jin, Atomically Precise Metal Nanoclusters: Stable Sizes and Optical Properties, Nanoscale, 2015, 7, 1549–1565, 10.1039/c4nr05794e.
- K. L. D. M. Weerawardene and C. M. Aikens, Effect of Aliphatic versus Aromatic Ligands on the Structure and Optical Absorption of Au20(SR)16, J. Phys. Chem. C, 2016, 120, 8354–8363, DOI:10.1021/acs.jpcc.6b01011.
- C. M. Aikens, Electronic Structure of Ligand-Passivated Gold and Silver Nanoclusters, J. Phys. Chem. Lett., 2011, 2(2), 99–104, DOI:10.1021/jz101499g.
- C. M. Aikens, Geometric and Electronic Structure of Au25(SPhX)18− (X = H, F, Cl, Br, CH3, and OCH3), J. Phys. Chem. Lett., 2010, 1, 2594–2599, DOI:10.1021/jz1009828.
- T. van Leeuwen, A. S. Lubbe, P. Štacko, S. J. Wezenberg and B. L. Feringa, Dynamic Control of Function by Light-Driven Molecular Motors, Nat. Rev. Chem., 2017, 1, 0096, DOI:10.1038/s41570-017-0096.
Footnotes |
| † This work is dedicated to Professor Jean-François Halet on the occasion of his 60th Birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/C9QI00513G |
| This journal is © the Partner Organisations 2019 |