Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diazido platinum(IV) complexes for photoactivated anticancer chemotherapy
Huayun
Shi
 ,
Cinzia
Imberti
,
Cinzia
Imberti
 and
Peter J.
Sadler
and
Peter J.
Sadler
 *
*
Department of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: p.j.sadler@warwick.ac.uk
First published on 30th May 2019
Abstract
Diazido Pt(IV) complexes with a general formula [Pt(N3)2(L)(L′)(OR)(OR′)] are a new generation of anticancer prodrugs designed for use in photoactivated chemotherapy. The potencies of these complexes are affected by the cis/trans geometry configuration, the non-leaving ligand L/L′ and derivatisation of the axial ligand OR/OR′. Diazido Pt(IV) complexes exhibit high dark stability and promising photocytotoxicity circumventing cisplatin resistance. Upon irradiation, diazido Pt(IV) complexes release anticancer active Pt(II) species, azidyl radicals and ROS, which interact with biomolecules and therefore affect the cellular components and pathways. The conjugation of diazido Pt(IV) complexes with anticancer drugs or cancer-targeting vectors is an effective strategy to optimise the design of these photoactive prodrugs. Diazido Pt(IV) complexes represent a series of promising anticancer prodrugs owing to their novel mechanism of action which differs from that of classical cisplatin and its analogues.
1. Introduction
The treatment of cancer, a group of diseases involving uncontrolled growth and spread of cells and representing the leading cause of death globally, remains a major challenge.1 Phototherapy, consisting of photodynamic therapy (PDT) and photoactivated chemotherapy (PACT), has attracted much attention in cancer treatment due to its high spatial-temporal controllability and minimal invasiveness. The mechanism of action of clinically approved PDT is based on the combination of a photosensitiser, light and oxygen.2 High oxygen dependence limits the application of PDT since the oxygen concentration in hypoxic tumours is low. PACT, in contrast to PDT, is an oxygen-independent phototherapy, which involves chemical changes of prodrugs upon irradiation and provides a new avenue in anticancer drug development.3,4The serendipitous discovery of the antiproliferative properties of cisplatin in 1968 by Rosenberg et al. stimulated a wide research for other platinum-based anticancer drugs.5,6 Platinum drugs are now used to treat over 40% of all cancer patients in chemotherapy since cisplatin was approved by FDA in 1978 to treat testicular and ovarian cancers.7–10 Several classes of platinum anticancer agents, as summarised in Table 1, have been investigated in the past decades and extensively reviewed in recent publications.11–21 Compared with Pt(II) complexes,22 Pt(IV) complexes with d6 electronic configuration are more kinetically inert to ligand substitution, and more stable under physiological conditions.14,23 Pt(IV) complexes can be reduced by bio-reductants (e.g. GSH, ascorbic acid, cysteine) to form active Pt(II) species through which they exert their anticancer activity.24–26 Although some Pt(IV) complexes entered clinical trials, none of them has been approved for clinical use.27–32
| Pt complexes | Mechanism of action | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Cisplatin-like Pt(II) complexes | Hydrolysis, covalent DNA binding | FDA approved, high cure rate | Poor pharmacokinetics, dose-limiting side effects, restricted anticancer spectrum, lack of selectivity, high incidence of resistance | 5 and 6 |
| Pt(II) complexes different from cisplatin | Covalent or non-covalent DNA binding | Overcome cisplatin resistance | Limited selectivity, inherent systemic toxicity | 16–19 |
| Photosensitive Pt(II) complexes | Excitation of triplet oxygen (PDT) | Good photoselectivity, overcome cisplatin resistance | Oxygen dependence, limited λex wavelength range | 20 and 21 |
| Photoactive Pt(II) complexes | Photoactivation to form cytotoxic Pt(II) species (PACT) | Oxygen independence | High dark cytotoxicity, low photocytotoxic indices | 45–53 |
| Chemically reductive Pt(IV) complexes | Reduction by bio-reductants | Easy modification, improved selectivity and cellular accumulation | Poor stability before entering cells | 30–32 |
| Photoactive Pt(IV) complexes | Photoreduction to release cytotoxic Pt(II) species and radicals (PACT) | Oxygen independence, good dark stability, overcome cisplatin resistance, spatial and temporal selectivity, easy modification | Low photocytotoxic indices, short λex wavelength range, limited light penetration | 62, 68, 77 and 81 |
Notably, photodecomposition is a prominent feature of Pt(IV) complexes, suggesting their potential as PACT prodrugs.15,33–35 Transition metal azido complexes are usually light sensitive.36,37 The first photoreductive Pt(IV) complex with azide ligands reported by Vogler et al. in 1978 was trans-[Pt(N3)2(CN)4]2−.38 Upon irradiation with UVA at 302 nm, the wavelength of its maximum electronic absorbance, assigned as a ligand-to-metal (N3 → Pt) charge-transfer transition, trans-[Pt(N3)2(CN)4]2− was converted to [Pt(CN)4]2−via the formation of two azidyl radicals without a Pt(III) intermediate. The unstable azidyl radicals released nitrogen gas, preventing the re-oxidation of the platinum centre.36 Another azido Pt(IV) complex [Pt(N3)6]2− was found to undergo photoreduction, yielding [Pt(N3)4]2− and even metallic Pt, accompanied by the release of nitrogen gas.39,40 These early studies provided a basis for our group to develop diazido Pt(IV) complexes that are stable in the dark, and exhibit promising cytotoxicity upon irradiation.11,15,34,35,41,42
In this review, our discussion begins with a brief introduction to photoactivated chemotherapy and focuses on the development of photoactive diazido Pt(IV) complexes, including the design of new complexes, their photochemistry and photobiology, mechanism of action, derivatisation of axial ligands and conjugation to nanoparticles to improve photocytotoxicity and selectivity. We give a comprehensive description of diazido Pt(IV) complexes and consider feasible strategies for their future clinical use.
2. Photoactivated chemotherapy
PACT relies on light-mediated chemical changes, transforming an inactive prodrug into an active agent upon irradiation.3,4,43,44 Platinum-based PACT anticancer drugs can be activated via different mechanisms as described below.• Photoreduction: Pt(IV) complexes can be reduced upon irradiation to release cytotoxic Pt(II) species and ligands.34,41
• Photosubstitution: Pt(II) complexes with photolabile ligand (e.g. curcumin) undergo ligand dissociation followed by solvent substitution.45–49
• Photocleavage of ligand: Photon absorption by the metal centre can result in the cleavage of organic bonds, (e.g. C–N, N–O) in photosensitive o-nitrobenzyl alcohol derivatives coordinated to platinum.50,51
• Photoswitching: Pt(II) complexes bridged by a diarylethene ligand change the configuration of the ligand upon irradiation to alter the cytotoxic properties.52,53
Although PACT is often compared with PDT since they both require light to activate relatively non-toxic drugs, these two classes of treatments present many differences.4 Generally, PACT agents decompose upon irradiation, while PDT agents are photostable. Notably, the oxygen-independent mechanism of action of PACT agents improves their efficiency in hypoxic tumours and represents a major advantage of PACT over PDT.
However, the development of PACT is still in its infancy, and the current performance of PACT complexes is not competitive with PDT photosensitisers.4 Some photosensitisers in clinical trials exhibit extremely high photocytotoxicity indices (photo-cytotoxicity/dark-cytotoxicity, e.g.TLD-1433, up to 105),54 and some can be excited by near infrared (NIR) irradiation (e.g.WST-09, 763 nm).55 In contrast, the highest reported photocytotoxicity index of PACT agents is 1880,56 and the longest excitation wavelengths reported for simple metal complex-based PACT agents are in the red region, which limits light penetration into tissue to a depth of ca. 5 mm.57–59 To enter clinical trials, more effort needs to be invested into the preclinical development of new PACT complexes.
3. Development of photoactive Pt(IV) complexes
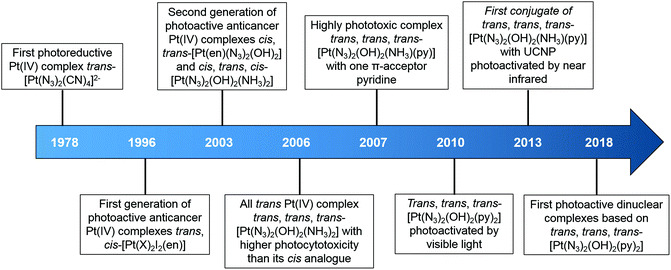
In general, photoactive Pt(IV) complexes contain axial, non-leaving, and leaving ligands. Axial ligands can be used to tune the physical and chemical properties of the complexes without altering the structure of the active pharmacophore that is ultimately released.60 Non-leaving ligands do not significantly alter the reduction potential of Pt(IV) complexes and can be varied to change their properties without reducing the dark stability.61 Leaving ligands play key roles in photoactive Pt(IV) complexes. Two main classes of Pt(IV) complexes, namely diiodo- and diazido-Pt(IV) complexes, have been investigated as photoactive Pt(IV) anticancer prodrugs (Scheme 1).Diiodo-Pt(IV)-ethylenediamines bearing various axial ligands (Cl, OH, OCOCH3, OSO2CH3, or OCOCF3) were reported by Kratochwil et al. in 1996 as the first-generation photoactive Pt(IV) complexes with a general formula trans, cis-[Pt(X)2I2(en)] (Fig. 1).62–66 Iodide is a weak field ligand with a low optical electronegativity,67 and thus the LMCT bands of these complexes are centred at the relatively longer wavelength of ca. 400 nm with a tail extending out into the visible range.62 As a result, all of these complexes are photolabile to light λirr > 375 nm. However, their application is limited due to their poor dark stability, which might be due to facile cellular bio-reduction. For example, electron donation to Pt(IV) can occur through thiolate attack on iodide ligands.65
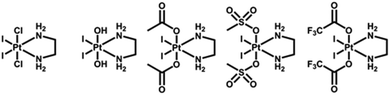
The second-generation photoactive Pt(IV) complexes with azide ligands, were developed later to achieve better dark stability and photobiological properties with a general formula [Pt(N3)2(L)(L′)(OR)(OR′)] (Fig. 2, Table 2). The first diazido-Pt(IV) complexes, cis, trans-[Pt(en)(N3)2(OH)2] (1) and cis, trans, cis-[Pt(N3)2(OH)2(NH3)2] (2) were reported in 2003.68 Cytotoxicity studies showed that these complexes caused very low inhibition of growth in the dark, but significantly enhanced cytotoxicity upon irradiation in both 5637 bladder cells and cisplatin-resistant 5637 bladder cells, even though to a less extent than cisplatin.69
| Complex | Non-leaving ligands | LMCT band λmax nm−1 (N3 → Pt) | λ irr/nm | Cell line | IC50 valuesa/μM | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | cis/trans | Light | Dark | PI | |||||
| a Note that IC50 values were obtained via different methods/conditions. PI = Photocytotoxicity index. | ||||||||||
| [Pt(N3)2(CN)4]2− | CN | CN | t | 302 | 302 | — | — | — | — | 38 |
| [Pt(N3)6]2− | — | — | 308 | 314 | — | — | — | — | 39 and 40 | |
| 1 | en | c | 315 | 365 | 5637 | 63.0 ± 20.2 | 440 ± 143 | 7.0 | 68 and 69 | |
| 365 | 5637-CDDP | 79.8 ± 16.6 | >200 | >2.5 | ||||||
| 2 | NH3 | NH3 | c | 256 | 365 | 5637 | 49.3 ± 28.1 | 357 ± 81 | 7.2 | 68 and 69 |
| 365 | HaCaT | 169.3 | >287.9 | >1.7 | 74 | |||||
| 3 | NH3 | NH3 | t | 285 | 365 | HaCaT | 121.2 | >287.9 | >2.4 | 70 and 74 |
| 4 | NH3 | MA | t | 286 | 365 | HaCaT | 65.6 | >276.8 | >4.2 | 74 and 76 |
| 5 | NH3 | Py | c | 258 | 365 | HaCaT | 100.9 | >244.4 | >2.4 | 74 |
| 6 | NH3 | Py | t | 289 | 365 | HaCaT | 6.8 | >244.4 | >35.9 | 74 and 77 |
| 7 | NH3 |
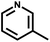
|
c | 259 | 365 | HaCaT | >236.2 | >236.2 | — | 74 |
| 8 | NH3 |
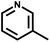
|
t | 289 | 365 | HaCaT | 22.0 | 144.1 | 6.6 | 74 and 76 |
| 9 | NH3 |
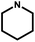
|
t | 288 | 366 | 5637 | 29.17 ± 2.23 | >80 | >2.7 | 79 |
| 10 | NH3 |
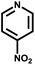
|
t | 287 | 365 | — | — | — | — | 75 |
| 11 | Py | MA | t | 289 | 365 | HaCaT | 2.6 | >236.3 | >90.8 | 80 |
| 420 | HaCaT | 14.7 | >236.3 | >16.0 | ||||||
| 12 | Tz | MA | t | 289 | 365 | HaCaT | 3.5 | >232.9 | >66.5 | 80 |
| 420 | HaCaT | 11.2 | >232.9 | >20.7 | ||||||
| 13 | Py | Py | t | 294 | 365 | HaCaT | 1.4 | >212.3 | >151.6 | 81 |
| 420 | HaCaT | 9.5 | >212.3 | >22.3 | ||||||
| 365 | A2780 | 1.4 | >212.3 | >151.6 | ||||||
| 365 | A2780cis | 14.5 | >212.3 | >14.6 | ||||||
| 14 | Bpy | c | 304/315 | 365 | — | — | — | — | 83 | |
| 15 | Phen | c | 300 | 365 | — | — | — | — | 83 | |
Intriguingly, the all trans photoactive Pt(IV) prodrug, trans, trans, trans-[Pt(N3)2(OH)2(NH3)2] (3), exhibited higher aqueous solubility and a more intense and red-shifted LMCT band compared with its cis isomer 2.70 Higher photocytotoxicity induced by 3 upon irradiation (as toxic as cisplatin) was determined in cancer cells, which was in contrast to the early structure–activity relationship for classical anticancer platinum complexes that the cis geometry was more favourable.71–73 Comparisons between other diazido Pt(IV) complexes incorporating a wide range of aliphatic and aromatic amines confirmed the higher potency of the trans configuration.74,75
The introduction of a methyl substituent into an ammonia ligand improved the photocytotoxicity of the Pt(IV) complexes trans, trans, trans-[Pt(N3)2(OH)2(NH3)(MA)] (4, MA = methylamine).74,76 Furthermore, replacement of an ammine ligand in 3 by a π-acceptor pyridine (Py) ligand resulted in the highly phototoxic complex trans, trans, trans-[Pt(N3)2(OH)2(NH3)(Py)] (6) that showed 13–80× higher photocytotoxicity compared with cisplatin, and 15× higher towards cisplatin-resistant human ovarian A2780cis cells.77 Notably, 6 is the only simple diazido-Pt(IV) prodrug that has been tested in vivo. Two of seven nude mice bearing OE19 oesophageal cancer xenografts treated with 6 at low dose with short irradiation times (420 nm, 100 J cm−2, 2 × 30 min) survived at 35 days, while none of the control mice without drug or irradiation survived, and the complex was well tolerated.78 Encouraged by the success of 6, a series of ligands was introduced to replace one or both of the ammine ligands. The replacement of pyridine by 3-methylpyridine (8),74,76 piperidine (9),79 or 4-nitro-pyridine (10)75 did not result in significant differences. Higher cytotoxicity of complexes with methylamine trans, trans, trans-[Pt(N3)2(OH)2(MA)(Py)] (11) and trans, trans, trans-[Pt(N3)2(OH)2(MA)(Tz)] (12, Tz = thiazole) was attributed to the high stability of Pt–MA bonds.80 In addition, the Tz-containing analogue 12 was more effective towards the cisplatin resistant cell lines.
The key role of pyridine ligands in the high phototoxicity and the mechanism of action different from cisplatin promoted the development of a novel diazido-Pt(IV) complex with two pyridine ligands, trans, trans, trans-[Pt(N3)2(OH)2(Py)2] (13).81 Upon irradiation with UVA or visible blue light, 13 exhibited significant toxicity toward a number of human cell lines with high phototoxicity indices. Dinuclear complexes based on 13 also displayed photodecomposition with blue light irradiation.82
Diazido-Pt(IV) complexes incorporating π-conjugated bidentate diimine ligands trans, cis-[Pt(2,2′-Bpy)(OAc)2(N3)2] (14, 2,2′-Bpy = 2,2′-bipyridine) and trans, cis-[Pt(Phen)(OAc)2(N3)2] (15, Phen = phenanthroline) exhibited greater absorption at longer wavelengths compared with previously reported diazido-Pt(IV) complexes.83 Their photodecompositions were observed upon irradiation with both UVA and visible green light.
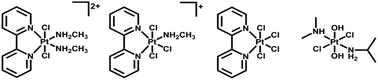
Other than iodide and azide, chloride is also a potential candidate as leaving ligand for photoactive Pt(IV) complexes. Upon irradiation, photoreduction to Pt(II) species was observed for trans-[PtCl2(2,2′-Bpy)(MA)2]Cl2, mer-[PtCl3(2,2′-Bpy)(MA)]Cl, [PtCl4(2,2′-Bpy)], and trans, trans, trans-[PtCl2(OH)2(DMA)(IPA)] (Fig. 3, DMA = dimethylamine, IPA = isopropylamine).84–86 Some Pt(IV) complexes with chloride ligands, such as oxoplatin, even though not photoactive on their own, exhibit photorelease of Pt(II) species when conjugated to specific nanoparticles.87,88
4. Photochemistry and photobiology of diazido Pt(IV) complexes
4.1. Photodecomposition pathways
Photodecomposition of diazido Pt(IV) complexes mainly relies on the reduction of Pt(IV) and the release of azide ligands triggered by light (Fig. 4a).38 The strong absorbance, assigned to a dissociative LMCT band (N3 → Pt), is a key transition in photodecomposition.70,74,75 Upon irradiation, a rapid decrease in intensity of the LMCT band can be observed for diazido Pt(IV) complexes due to the release of azide ligands (Fig. 4b and c).38,41 The wavelength of the LMCT band is affected significantly by the overall configuration of Pt(IV).70,74,75,89 In general, complexes with cis-{PtIV(N3)2} motifs exhibit LMCT bands at shorter wavelength (ca. 260 nm), while those of trans isomers are red-shifted by 30 nm (ca. 290 nm) (Table 2).74 However, complexes with bidentate chelating non-leaving ligands display LMCT bands at longer wavelength (ca. 300 nm) than diazido Pt(IV) complexes with only monodentate ligands, despite of their cis configuration.68,83,89 Electronic transitions for cis isomers tend to arise from transitions from orbitals of the azide ligands to metal d orbitals, whereas those for all-trans isomers are more likely to arise from both axial and azide ligands to the metal d orbitals, and thus lead to different photoproducts.75 The electron-donating and steric properties of non-leaving ligands are also important factors which affect the LMCT band. The replacement of NH3 by a π-acceptor pyridine ligand results in a red-shift of the intense LMCT band (ca. 5 nm for each substitution).70,77,81 The substituents on pyridine ligands affect the maximum absorption slightly.75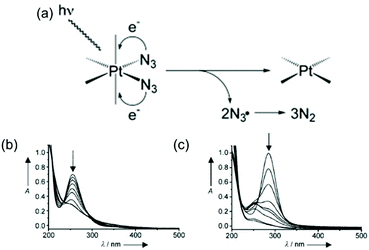 | ||
| Fig. 4 (a) A possible mechanism for the photoreduction of diazido-Pt(IV) complexes;91 UV-vis spectral changes for (b) 2 and (c) 3 in aqueous solution upon UVA irradiation.70 Adapted from ref. 91 and 70. | ||
The low-intensity transitions (the “tail”) that have mixed dissociative 1LMCT/1IL (IL = interligand) character might account for the photoactivity induced by longer wavelength activation,75,81 which is a major challenge in the development of new diazido Pt(IV) complexes. The low-intensity transitions have significant contributions from the LUMO orbital that is strongly σ-antibonding towards the two Pt–N3 bonds.81 Light excitation to populate this orbital induces elongation of the Pt–N3 bond, and eventually leads to azide release.81 The weak absorption of 13 in the blue region (414 nm) might be responsible for its photodecomposition with visible light.81
Much effort has been devoted to the investigation of the photodecomposition pathways of the most promising diazido Pt(IV) complexes. The early studies suggested that the photodecomposition involved the release of two azide ligands as N3˙ radicals, which can combine to form N2 molecules.38 However, during further investigation of diazido Pt(IV) complexes, different pathways have been proposed depending on the configuration of Pt(IV), non-leaving ligands, and the solution environment.
1D 14N{1H} NMR spectra of 15N-labelled 2 with two 15NH3 in acidic aqueous solution (pH = 5.1) upon irradiation with UVA (365 nm) suggested the formation of N2 and unlabelled free NH3, with a pH increased to 10.7 and O2 liberation detected.90 In addition, the major Pt(II) species detected was assigned to trans-[Pt(NH3)2(H2O)2]2+, indicating photoisomerisation with photoreduction. Nitrene intermediates generated during the photodecomposition were trapped by dimethyl sulphide (DMS), which suggested a possible new mechanism.90 However, the photodecomposition of 2 in PBS involved the photosubstitution of at least one azide ligand by H2O/OH and the release of azide ions (Fig. 5).91 Ammonia ligands were also released from Pt(IV) as confirmed by NMR with an increase in pH. After replacement of both azides (or even ammonia ligands) by H2O/OH, photoreduction may occur by one-electron transfers from each of two hydroxide groups coordinated to the Pt(IV) leading to some species containing trans-{Pt(OH)2} motifs and hydroxyl radicals. DFT and TD-DFT calculations demonstrated the theoretical possibility of the replacement of both NH3 and N3 groups trans to each other by H2O/OH.92
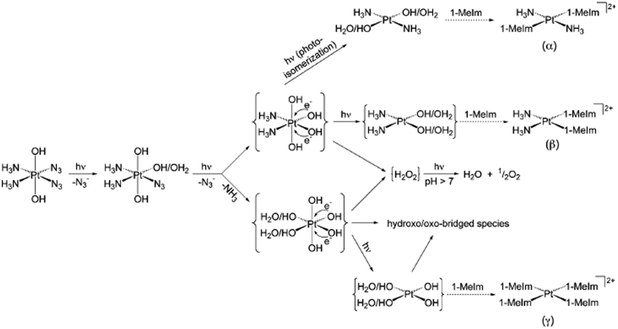 | ||
| Fig. 5 Possible pathways for photodecomposition and photoreactions of complex 2 with 1-methylimidazole in PBS. Adapted from ref. 91. | ||
The photodecomposition pathways for 3 in PBS and acidic solution are similar to that of its cis isomer 2, in which the azide ion was detected in PBS, while N2 was found in acidic solution.93Trans Pt(II) species, O2 and free ammonia were detected as photoproducts of 3 with a subsequent increase in pH in both basic and acidic solution. However, the trans isomer 3 gave fewer types of Pt species in acidic solution, but more minor side-products and less hydroxo-/oxo-bridged species compared to its cis isomer 2.93 In addition, no photoisomerisation was observed in the photodecomposition of 3.
Even though azidyl radicals are regarded as important cytotoxic photoproducts of diazido Pt(IV) complexes, they were not considered further in the early work.90–93 A recent study on the first stage of photodecomposition using ultrafast kinetic spectroscopy and nanosecond laser flash photolysis suggested that the photodecomposition of both cis2 and trans3 is a chain process beginning with the replacement of one azide ligand by a water molecule and the release of azidyl radicals.94 Two successive Pt(III) intermediates participated in chain initiation and [PtIII(NH3)2(OH)2]+ was a chain carrier.
Trans-[Pt(OH)4(15NH3)(Py)] and trans-[Pt(N3)(OH)3(15NH3)(Py)] were detected as photoproducts of 6 using 15NH3 and 1D 1H and 2D [1H, 15N] HSQC NMR spectroscopy, indicating the release of azides.77 In contrast, no evidence of pyridine release was found. Furthermore, very little reduction to Pt(II) species was detected by NMR. Consistently, [Pt(OH)2NH3(Py)] and [Pt(OH)(N3)(NH3)(Py)] at low concentration were detected by LC-MS.95 In addition, [Pt(N3)3(OH)(NH3)(Py)] was observed by LC-MS as a photosubstitution product of 6 as well as azide ions, indicating the release of azide ligands.95 No platinum species without pyridine were detected by LC-MS, in accordance with NMR data. However, no detailed photodecomposition pathway has been proposed for 6 so far. For its piperidine analogue 9, only [Pt(OH)2(piperidine)NH3] was detected as a product by LC-MS, suggesting an effect of the heterocyclic ring on the photodecomposition pathways.96 The MA analogue of 6, 11 produced N2, N3−, N3 radicals and 1O2 upon irradiation, with Pt–nitrene intermediates involved in the pathway (Fig. 6).97
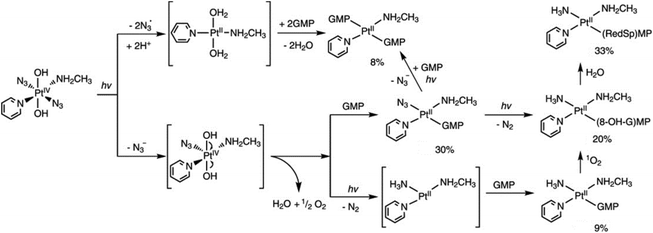 | ||
| Fig. 6 Possible pathways for photodecomposition and photoreactions of 11 with 5′-GMP upon irradiation with UVA. Adapted from ref. 97. | ||
Comprehensive spectroscopic studies of the photodecomposition of the most promising diazido Pt(IV) complex 13 have been performed by Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) and NMR.81,98 ATR-FTIR revealed the binding of water to platinum in the final product, which was assigned as trans-[PtII(Py)2(H2O/OH)2] fitted by Multi-Curve Resolution Alternating Least Squares (MCR-ALS). Trans-[PtII(N3)(Py)2(H2O/OH)] was detected as an intermediate, implying release of at least one hydroxyl radical and one azidyl radical or two hydroxyl radicals during the photoreduction.98 No pyridine release was detected by 1H NMR, in contrast to NH3 complexes, and might contribute to its higher potency.81
4.2. Photoreactions with important biomolecules
The cytotoxicity of diazido Pt(IV) complexes is mediated by the photoreduction products, including Pt(II) species, azidyl radicals and ROS, and their interactions with biomolecules, including nucleotides, DNA, amino acids, peptides and proteins.35,41The RNA monomer nucleotide guanosine monophosphate (5′-GMP) is often used as a model for nucleobase guanine, considered to be the major target of Pt anticancer drugs on DNA.99,100 Unlike Pt(II) complexes, diazido Pt(IV) complexes do not interact with 5′-GMP in the dark, but only upon irradiation, forming mono- and/or bis-GMP adducts. A bis-GMP adduct [Pt(en)(GMP-N7)2]2+ was detected by NMR when 1 reacted with two mol. equiv. of 5′-GMP upon irradiation with visible light (457.9 nm), while [Pt(en){d(GpG)-N71,N72}]2+ was the only major photoproduct of 1 in the presence of one mol. equiv. of d(GpG).68 Similar results were found for 2 and 3.68,70 Interestingly, trans3 was able to form a bis(5′-GMP) adduct under UVA irradiation at a faster rate than transplatin.70 In addition, the photodecomposition of 3 was accelerated in the presence of 5′-GMP, and a few Pt-GMP adducts were even detected upon red light irradiation.70 For 6, the Pt(II) mono-GMP adduct [Pt(N3)(NH3)(5′-GMP)(Py)]+ was detected as the initial main photoproduct from reaction with 5′-GMP, while the bis-5′-GMP adduct trans-[Pt(NH3)(Py)(5′-GMP)2]2+ became predominant at longer irradiation times.77 Unexpectedly, guanine photooxidation by 11 was observed via a mechanism involving 1O2, while the NH3 ligand in the adducts probably arose from the formation of a Pt–nitrene intermediate (Fig. 6).97 Both mono- and bis-GMP adducts (trans-[Pt(N3)(Py)2(5′-GMP)]+ and trans-[Pt(Py)2(5′-GMP)2]2+) were detected as photoproducts of 13 and 5′-GMP by NMR, LC-MS and ATR-FTIR spectra.81,98
DNA platination photoinduced by 1 with rb = 0.01 (rb = Pt coordinated per nucleotide residue) gave a preference for GG sequences, forming bifunctional GG adducts, which is similar to cisplatin.101 Intriguingly, photoactivated 1 and 2 formed GG adducts more rapidly than cisplatin.69 Transcription mapping revealed similar major stop sites in a DNA fragment treated with 6 and irradiation or with transplatin, while the level of DNA adducts formed by irradiated 6 was significantly higher than that by cisplatin and transplatin.77 DNA interstrand cross-links and DNA–protein cross-links were also induced by 6 with light. Remarkably, a plasmid treated with 6 and UVA exhibited considerably lower levels of damage-induced DNA repair synthesis than those with cisplatin. Significantly larger unwinding angles and a higher percentage of interstrand cross-links were observed when DNA was treated with 11 or 12 compared to cisplatin.80 The nature of DNA lesions induced by 13 upon irradiation was expected to be different from those induced by cisplatin.81 Irreversible DNA coordination was observed for 13 with light, including ca. 12% interstrand (intramolecular) cross-links, ca. 37% monofunctional adducts, and ca. 51% intrastrand cross-links.102 The interstrand cross-links formed by 13 and light were characterised, and the structure of a trans-{Pt(Py)2}2+-DNA showing a bend toward the minor groove, a global bend of ca. 67° and an unwinding of ca. 20° was simulated by computational studies (Fig. 7).103
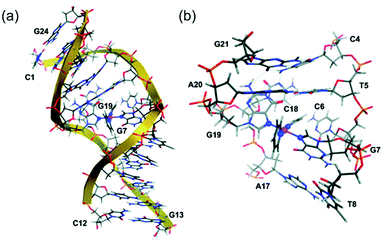 | ||
| Fig. 7 (a) The average ligand field molecular dynamics (LFMD) structure of trans-{Pt(Py)2}2+-DNA derived from simulations; (b) an expanded view of the platinum-binding site showing the G7–G19 interstrand cross-link within the DNA duplex dodecamer, d(5′-C1C2T3C4T5C6G7T8C9T10C11C12-3′)·d(5′-G13G14A15G16A17C18G19A20G21A22G23G24-3′). Adapted from ref. 103. | ||
Other than nucleotides and DNA, amino acid, peptides and proteins are also targets for Pt(II) species released from diazido Pt(IV) complexes. The photoactivated platinum centre of 2 can also react with dimethyl sulphide, a thioether related to that in the side chain of the amino acid methionine (Met), in acidic solution. The nitrene intermediate formed dimethylsulfilimine adducts that can undergo a Stevens-like rearrangement in which the Pt–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SMe2 group becomes Pt–N(H)–CH2–SMe, giving a N-(methylthiomethylene)amido derivative.90 The derivative can release two hydroxyl radicals upon irradiation.90 The photoreaction between 2 and 1-methylimidazole, a histidine (His) side chain analogue, in PBS (Fig. 5) gave 6 different Pt-coordinated 1-methylimidazole species, suggesting it can bind to proteins.91
SMe2 group becomes Pt–N(H)–CH2–SMe, giving a N-(methylthiomethylene)amido derivative.90 The derivative can release two hydroxyl radicals upon irradiation.90 The photoreaction between 2 and 1-methylimidazole, a histidine (His) side chain analogue, in PBS (Fig. 5) gave 6 different Pt-coordinated 1-methylimidazole species, suggesting it can bind to proteins.91
Azidyl radicals are potentially key species, which might kill cancer cells by oxidative attack. Azidyl radicals generated by 13 can be trapped by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and detected using EPR (Fig. 8a).104 However, in the presence of Trp, the azidyl radicals were quenched, which reduced the photocytotoxicity of 13.104 The resulting tryptophan (Trp) radicals have been trapped by 2-methyl-2-nitrosopropane (MNP) and such radicals might be involved in the cytotoxicity towards cancer cells (Fig. 8b).105 Background signals from di-tert-butyl nitroxide (DTBN) radicals arose from MNP upon irradiation. The Trp containing peptide pentagastrin also quenched azidyl radicals (Fig. 8c), although not as efficiently as Trp.105 The antioxidant melatonin (MLT) quenched azidyl radicals and formed MLT radicals in a similar way to Trp.105 In contrast, other amino acids, including Tyr (tyrosine) and His, were unable to quench azidyl radicals despite of their important roles in electron transfer.104,105
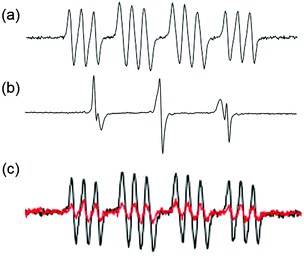 | ||
| Fig. 8 EPR spectra of 13 in PBS upon irradiation in the presence of spin-traps (a) DMPO for N3˙ radicals; (b) Trp + MNP for Trp˙ radicals (and background DTBN˙ radicals); (c) pentagastrin + DMPO for N3˙ radicals (red: with pentagastrin; black: without pentagastrin). Adapted from ref. 105. | ||
A combined attack on peptides by photoactivated 13, involving sequence-dependent platination and radical mechanisms, was observed using UHR-FT-ICR MS and EPR.106 Two model peptides with amidated C-termini, Substance P (SubP) and [Lys]3-Bombesin (K3-Bom), were treated with 13 and irradiation. SubP gave rise to only mono-platinated adducts with different amino acids, while K3-Bom showed both mono- and di-platinated adducts with a preference for His. In contrast, oxidation of SubP occurred only at Met, whereas oxidation of both Met and Trp was observed for K3-Bom. Thioredoxin (Trx) is an important enzyme in the redox signalling pathway and is usually overexpressed in tumour cells.107 Platination of His, Glu (glutamic acid), and Gln (glutamine) residues of Trx and oxidation of Met, Trp, and the Cys catalytic sites induced by 13 upon irradiation can inhibit the activity of Trx enzyme and Trx system, and further increase the cellular ROS level.108
4.3. Effects on cancer cellular components and pathways
Photoreactions between photoactivated diazido Pt(IV) complexes and important biomolecules can result in the dysfunction of cellular components, changes to cellular morphology and disruption of cellular pathways, and eventually cell death.The ability to damage DNA in intact cells has been investigated for both cis2 and trans3 using comet assays.70 Both complexes produced DNA cross-links in living cells upon irradiation and the effects were similar to those produced by cisplatin. When 5637 bladder cells were treated with 2 in the presence of light, dramatic changes in the morphology of the cells occurred, including cellular shrinkage, loss of adhesion with neighbouring cells, a large amount of nuclear packing, and nuclei disintegration, suggesting a different mechanism of cell death compared to cisplatin (Fig. 9).69 None of these effects was observed without irradiation. Intriguingly, typical hallmarks of apoptosis, budding and cellular fragmentation, were not observed.
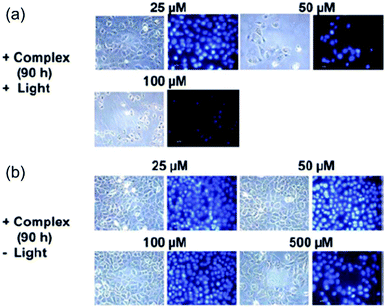 | ||
| Fig. 9 Fluorescence microscopy images of 5637 cells treated by 2 with and without irradiation. Adapted from ref. 69. | ||
Single-cell electrophoresis experiments indicated that different DNA damage in HaCaT keratinocyte cells was caused by 6 and light with limited inhibition of DNA migration compared to cisplatin.77 Also, p53 protein did not accumulate in cells, and caspase 3 activity was not detected when treated with 6 and irradiation, which was different from cells treated with cisplatin.77 An autophagic mechanism was envisaged for 6 based on a significant increase in LC3B-II level and a decreased p62 level in treated cells.78 Cell swelling and very little blebbing was seen for HL60 cells treated with irradiated 6.78 Notably, light activation was found to enhance the cellular accumulation of 6.79
Excellent nuclear DNA binding properties were observed for photoactivated 13 that was shown to be a potent inhibitor to stall RNA pol II for RNA synthesis.102 In contrast to cisplatin, photoactivated 13 did not produce fragmented or condensed nuclei, indicating a different mechanism.81
5. Derivatisation of photoactive diazido Pt(IV) complexes
The axial ligands (with arbitrary choice of OH as axial ligands) of photoactive Pt(IV) complexes can not only be released, similar to azide ligands, upon photoreduction to Pt(II), but also greatly affect the reduction potential of Pt(IV).66,75 Generally, Pt(IV) complexes with a lower reduction potential (depending on the axial ligand: I− > Cl− > OAc− > OH−) exhibit higher stability to reductants.60,66 Thus, the hydroxide ligands can enhance aqueous solubility and also stabilise the Pt(IV) oxidation state.109 Modification of axial ligands is a feasible method to improve the selectivity and cytotoxicity of diazido-Pt(IV) complexes.5.1. Multi-action diazido-Pt(IV) complexes
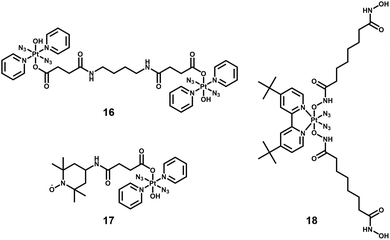
By combining photoactive Pt(IV) complexes with other anticancer active agents, such as stable radicals, enzyme inhibitors, and photosensitisers, multi-action prodrugs are obtained. Upon irradiation, these prodrugs release not only the reactive Pt(II) species and azidyl radicals, but also additional cytotoxic agents, enhancing the anticancer efficacy of the drugs. Owing to their different cellular targets and mechanism of action, combination of these cytotoxic species often results in a synergistic effect, producing greater cytotoxicity than that expected by simple addition of the two agents. Examples of diazido-Pt(IV) multi-action agents are summarised below.Dinuclear complexes such as 16, in which two molecules of 13 are bridged in an axial position by bisamide dicarboxylato linkers (Fig. 10) display similar photocytotoxicity towards cisplatin-resistant ovarian A2780cis and A2780 cells, and interestingly are relatively non-toxic toward normal cells (MRC-5 lung fibroblasts).82
The TEMPO radical has been conjugated to 13 since nitroxide radicals themselves can possess potent anticancer activity (17, Fig. 10).110 The presence of the TEMPO radical in the complex was confirmed by EPR spectroscopy. Upon irradiation with blue light (420 nm), azidyl and TEMPO radicals were released, accompanied by the formation of toxic Pt(II) species.
Suberoyl-bis-hydroxamic acid (SubH) is a histone deacetylase (HDAC) inhibitor, which exhibits a profound dose-dependent inhibition of cancer cell proliferation.111 Two SubH ligands have been attached to the axial positions of cis, trans-[Pt(N3)2(OH)2(tBu2bpy)] to generate a diazido-Pt(IV) complex, cis, trans-[Pt(N3)2(Sub)2(tBu2bpy)] (18, Fig. 10), that was stable in the dark.112 Complex 18 released SubH and cytotoxic Pt(II) species, which targeted similar DNA regions as cisplatin efficiently upon UVA irradiation. Photoactivated 18 exhibited significant cytotoxicity in cancer cells with a low resistance factor compared with cisplatin and its Pt(IV) analogues containing inactive axial ligands. This suggested a different mechanism of action involving inhibition of HDAC that allowed platinum species to access chromatin DNA, introduced effective steric blockage of RNA polymerase II and formed DNA adducts (e.g. interstrand cross-links).
Metal complexes with curcumin ligands exhibit photoinduced anticancer activity.113 Light activation of curcumin-loaded Dex-2 nanoparticles 19 led to instant production of ROS by curcumin and released Pt(II) species from 2 (Fig. 11), and gave rise to photocytotoxicity and in vivo antitumour efficacy with low systemic toxicity to KM mice bearing subcutaneous H22 murine hepatocarcinoma tumour.114
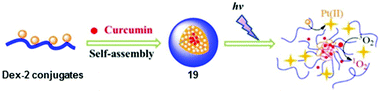 | ||
| Fig. 11 Preparation and photodecomposition of curcumin-loaded Dex-2 nanoparticles 19. Adapted from ref. 114. | ||
The amphiphilic oligomer Ce6-PEG-Pt(IV) (CPP, 20, Fig. 12) can self-assemble into micelles, and upconversion nanoparticles (UCNP) NaYbF4: Tm@CaF2 have been co-assembled to convert NIR into shorter wavelength irradiation that can induce decomposition of diazido-Pt(IV) complexes.115 Micelles as self-assembled nanoparticles with a hydrophilic corona and hydrophobic core have been widely used in anticancer drug delivery.116 Attachment of the photosensitiser Chlorin e6 to complex 2 resulted in an O2-self generating PACT-PDT agent, where the photosensitiser relied on the oxygen produced by photoactivation of the diazido-Pt(IV) fragment to supply the oxygen required for PDT in hypoxic tissues. Dramatically enhanced photocytotoxicity was observed for this PACT-PDT system in hypoxic tumour models.
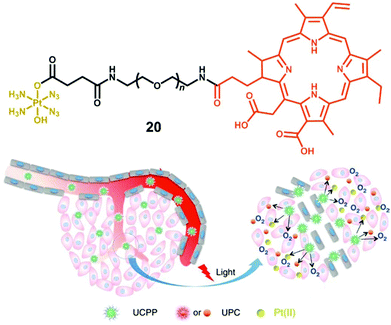 | ||
| Fig. 12 The amphiphilic oligomer 20 and the photodecomposition of UCPP in a tumour microenvironment. Adapted from ref. 115. | ||
The nanoparticle-based agent 21 has complex 13 loaded onto silica-coated UCNP to allow photoactivation with NIR, together with a fluorescent probe that can be selectively switched-on in apoptotic conditions (Fig. 13a).117 The probe included a fluorescence resonance energy transfer (FRET) pair, consisting of a far-red fluorescence donor Cy5 and a NIR quencher Qsy21, linked by a peptide sequence that can be recognised and cleaved by caspase-3, a key enzyme in apoptosis. Accordingly, when 21 was photoactivated by NIR irradiation, a chain reaction in A2780 ovarian cancer cells started on triggering apoptosis, thus activating caspase-3, which in turn cleaved the peptide-linker, switching on emission of the Cy5 dye and enabling imaging of apoptosis in living cells. Notably, when both peptide probe and Pt(IV) complex 13 were conjugated to human serum albumin protein (HSA, 22, Fig. 13b), instead of UCNP, improved photocytotoxicity and real-time imaging was also achieved upon UVA irradiation.118
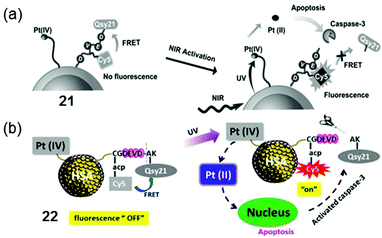 | ||
| Fig. 13 Photoactivation of conjugates 21 and 22 based on (a) UNCP117 and (b) HSA,118 respectively. Adapted from ref. 117 and 118. | ||
The nano-system 23, combining Yb/Tm-codoped UCNP and 6, exhibited better tumour inhibition under NIR irradiation than that under UV irradiation (Fig. 14).119 Notably, this nano-system functioned as a theragnostic agent, whose therapeutic action could be informed and guided by different imaging modalities, such as upconversion luminescence (UCL), magnetic resonance (MR) and computer tomography (CT).
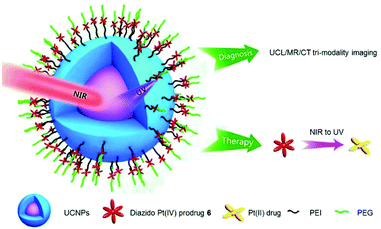 | ||
| Fig. 14 Schematic illustration of the photodecomposition of conjugate 23. Adapted from ref. 119. | ||
5.2. Targeted delivery of diazido-Pt(IV) complexes
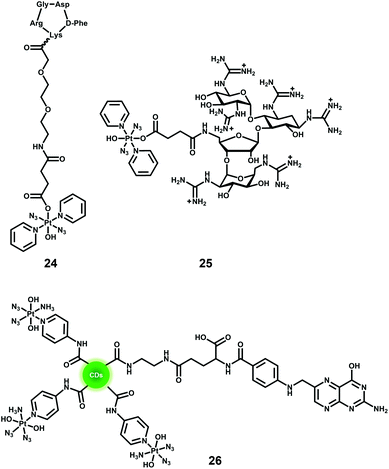
One of the major advantages of phototherapy over traditional chemotherapy is the ability to provide spatial and temporal control of the activation of prodrugs and their resulting cytotoxic activity. However, inefficient accumulation of photoactive prodrugs at the tumour site is still an issue and results in the need for higher concentrations to achieve therapeutic efficacy, increasing the risk of side effects. The conjugation of diazido Pt(IV) complexes with cancer-targeting vectors can improve their selectivity, increase their accumulation in cancer cells, and enable the prodrugs to be activated specifically within cancer cells.Overexpression of particular receptors on the surface of cancer cells provides an excellent strategy for the targeted delivery of diazido-Pt(IV) complexes by tethering them to ligands that can bind selectively to those receptors. The RGD sequence (–Arg–Gly–Asp–) can be selectively recognised by αvβ3 and αvβ5 integrins that are overexpressed on the surface of several tumour cells and related to tumour angiogenesis.120,121 The conjugate of photoactive Pt(IV) prodrug 13 and a cyclic RGD-containing peptide c(RGDfK) (24, Fig. 15) showed remarkably enhanced selectivity and increased cellular accumulation for cells overexpressing αvβ3 and αvβ5 integrins, although the IC50 values for irradiated cells were higher than those of its parent succinylated complex.122 Another example of targeting for complex 13 is its conjugate with guanidinoneomycin, which is a RNA-binding ligand and allows the complex to be taken up by cancer cells in a selective proteoglycan-dependent manner (25, Fig. 15).123 The photoproducts of Pt–guanidinoneomycin conjugate 25 with 5′-GMP or 5′-dCATGGCT were similar to that of the parent complexes under the same conditions. Similar to the Pt–c(RGDfK) conjugate, the Pt–guanidinoneomycin conjugate exhibited an enhanced cellular uptake with a preference for the SK-MEL-28 malignant melanoma cell line due to the expression of negatively charged cell-surface proteoglycans.
Nanoparticle-based drug delivery systems have attracted particular attention due to their enhanced accumulation in tumour tissue through the enhanced permeation and retention (EPR) effect caused by the leaky nature of angiogenic blood vessels in solid tumours.88,124,125 The PtIV-N3-FA@CDs nanoplatform 26 (Fig. 15) consisted of carbon dots decorated with the photoactive diazido Pt(IV) prodrug cis, trans, cis-[Pt(N3)2(OH)2(NH3)(3-NH2-Py)] and folic acid (FA) molecules, and exhibited a preference for folate receptor FR-positive [FR(+)] human cervical HeLa cells over FR-negative [FR(−)]MCF-7 human breast tumour cells.126
Photo-responsive block copolymer (BCP) micelles have been widely investigated for their drug delivery applications in cancer therapy.127 The triblock copolymer methoxy-poly(ethylene glycol)-block-poly(ε-caprolactone)-block-poly-L-lysine, mPEG114-b-PCL20-b-PLL10 that included mPEG (n = 114), polycaprolactone (p = 20) and poly-L-lysine (q = 10) self-assembles in aqueous solution, with polycaprolactone and poly-L-lysine forming the core with Pt(IV) complex cis, trans-[Pt(1R,2R-DACH)(N3)2(OH)2] (DACH = 1,2-cyclohexanediamine) covalently encapsulated inside the hydrophobic core via an amide linkage (27, Fig. 16).128 The micelles exhibit greatly enhanced cellular accumulation and photocytotoxicity to SKOV-3 ovarian cancer cells compared with polymer-free prodrugs. Importantly, in vivo studies revealed that conjugation to micelles enhanced the blood circulation half-life of the Pt(IV) complex by 10-fold and displayed improved inhibition efficacy against H22 murine hepatocarcinoma with decreased systemic toxicity. Complex 3 was also attached to the biodegradable polymer mPEG114-b-PCL20-b-PLL10 that can self-assemble into micelles with the hydrophobic chain and Pt species as the core to protect Pt(IV) prodrugs from potential deactivation in blood circulation.129 The micelles 28 (Fig. 16) displayed comparable IC50 values to cisplatin upon irradiation and a resistance factor (IC50 ratio: A2780CDDP/A2780) 5× lower than cisplatin. When the same micelles incorporated sterically hindered 6, they were proved to be >100 times more effective than cisplatin upon UVA irradiation (29, Fig. 16).130
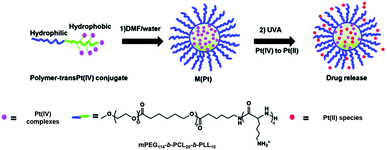 | ||
Fig. 16 The preparation and photodecomposition of micelles 27,12828,12929![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 assembled by mPEG114-b-PCL20-b-PLL10 polymer with diazido Pt(IV) complexes. Adapted from ref. 129. 130 assembled by mPEG114-b-PCL20-b-PLL10 polymer with diazido Pt(IV) complexes. Adapted from ref. 129. | ||
The photocleavable tri-block copolymers PEG-PUPt(N3)-PEG (PUPt(N3) is polyurethane with repeating 13 bricks) produced a photosensitive micelle 30 (Fig. 17).131 The in vivo experiments revealed that the irradiated micelle (430 nm) was 3–4× more effective to BALB/c nude mice bearing A549 xenografts than the prodrug and cisplatin with lowest body weight loss on day 27, suggesting lower systemic toxicity.
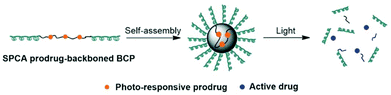 | ||
| Fig. 17 The preparation and photodecomposition of micelles 30 assembled by a tri-block copolymer with complex 13. Adapted from ref. 131. | ||
Complex 2-loaded amphiphiles with one lactose motif self-assembled into micelles 31, while the amphiphiles with two lactose motifs formed vesicles 32 instead (Fig. 18). These nanoparticles displayed photocytotoxicity with liver cancer-targeting ability in vivo. The platinum distribution in mice was determined by fluorescence, CT and ICP-MS.132
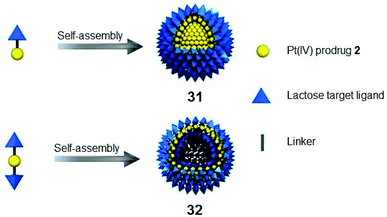 | ||
| Fig. 18 Cancer-targeting micelles 31 and vesicles 32. Adapted from ref. 132. | ||
For surface tumours, such as non-melanoma skin cancer, topical medication is an ideal way for precise localisation. G4K+ hydrogels are biocompatible polymers with flexibility and high water content that allow them to mimic natural tissues.133–135 Pt(IV)-based Pt-G4K+ B hydrogel 33 (Fig. 19) exhibited potent photocytotoxicity towards cisplatin-resistant A2780cis ovarian cancer cells while it displayed negligible cytotoxicity to MRC-5 normal fibroblast cells.136
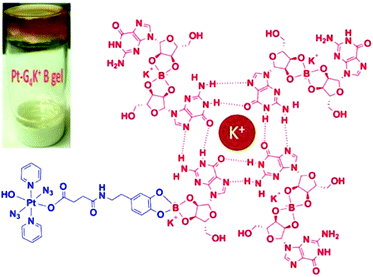 | ||
| Fig. 19 Schematic illustration of self-assembled Pt-G4K+B anticancer hydrogel 33. Adapted from ref. 136. | ||
6. Conclusions and prospects for clinical platinum photochemotherapy
Photoactive diazido-Pt(IV) complexes represent a new generation of platinum anticancer prodrugs that allow spatial and temporal control over their cytotoxic activity. They exhibit high dark stability, promising oxygen-independent photocytotoxicity, and novel mechanisms of action that allow them to circumvent cisplatin resistance. Modification of the prototype complexes (1–15), especially the axial ligands, improves their targeting ability and pharmacological properties. In this review, we have described the development of photoactive diazido-Pt(IV) complexes, including their cancer-targeting derivatives, and discussed their photodecomposition pathways, photoreaction with biomolecules, and their effect on cellular components and pathways.Three main features of an ideal diazido-Pt(IV) complex for PACT appear to be crucial and need to be considered in any further development of these agents. (1) In contrast to cisplatin, trans geometry is more favourable than cis for diazido-Pt(IV) complexes, owing to the more intense and red-shifted LMCT bands, faster photodecomposition rate and improved photocytotoxicity. (2) The replacement of NH3 by a π-acceptor pyridine ligand can not only red-shift the LMCT bands, but also enhance the cytotoxicity and introduce new mechanisms of action. Also, replacement of NH3 by an aliphatic amine (e.g. CH3NH2) improves photocytotoxicity. (3) Axial derivatisation of hydroxide/carboxylate ligands with cancer-targeting vectors or cytotoxic motifs provides an avenue for the design of new diazido-Pt(IV) complexes with improved selectivity, cellular accumulation and photocytotoxicity, longer activation wavelength, and convenient formulation.
In spite of the notable progress with research development of photoactive diazido-Pt(IV) complexes achieved so far, none of them has yet entered clinical trials. The achievement of longer wavelength activation for deeper tissue penetration, higher photocytoxicity indices, and tumour specific drug delivery remain major challenges to be further addressed. However, there are good prospects for use of such complexes for surface cancers such as bladder and oesophageal cancer. Hence there are encouraging signs that diazido-Pt(IV) complexes can provide novel PACT anticancer drugs with new mechanisms of action which can be effective against resistant cancers, so warranting further investigation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the MRC (grant G0701062), EPSRC (grants EP/G006792, EP/F034210/1, EP/P030572/1 to PJS), ERC (grant 247450 to PJS), a Chancellor's International PhD Scholarship from the University of Warwick (for HS), and the Wellcome Trust (209173/Z/17/Z, Sir Henry Wellcome Fellowship for CI).Notes and references
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2019, 69, 7–34 CrossRef PubMed.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- N. J. Farrer, L. Salassa and P. J. Sadler, Dalton Trans., 2009, 48, 10690–10701 RSC.
- S. Bonnet, Dalton Trans., 2018, 47, 10330–10343 RSC.
- B. Rosenberg, L. Van Camp and T. Krigas, Nature, 1965, 205, 698–699 CrossRef CAS PubMed.
- B. Rosenberg, L. Van Camp, J. E. Trosko and V. H. Mansour, Nature, 1969, 222, 385–386 CrossRef CAS PubMed.
- L. Kelland, Nat. Rev. Cancer, 2007, 7, 573–584 CrossRef CAS PubMed.
- S. C. Sweetman, Martindale: The complete drug reference, Pharmaceutical Press, London, 35th edn, 2007 Search PubMed.
- D. Wang and S. J. Lippard, Nat. Rev. Drug Discovery, 2005, 4, 307–320 CrossRef CAS PubMed.
- B. W. Harper, A. M. Krause-Heuer, M. P. Grant, M. Manohar, K. B. Garbutcheon-Singh and J. R. Aldrich-Wright, Chem. – Eur. J., 2010, 16, 7064–7077 CrossRef CAS PubMed.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, Chem. Rev., 2016, 116, 3436–3486 CrossRef CAS PubMed.
- L. Cai, C. Yu, L. Ba, Q. Liu, Y. Qian, B. Yang and C. Gao, Appl. Organomet. Chem., 2018, 32, e4228 CrossRef.
- I. Kostova, Recent Pat. Anti-Cancer Drug Discovery, 2006, 1, 1–22 CrossRef CAS.
- D. Gibson, J. Inorg. Biochem., 2019, 191, 77–84 CrossRef CAS PubMed.
- K. Mitra, Dalton Trans., 2016, 45, 19157–19171 RSC.
- M. Coluccia, A. Nassi, F. Loseto, A. Boccarelli, M. A. Mariggio, D. Giordano, F. P. Intini, P. Caputo and G. Natile, J. Med. Chem., 1993, 36, 510–512 CrossRef CAS PubMed.
- K. S. Lovejoy, R. C. Todd, S. Zhang, M. S. McCormick, J. A. D'Aquino, J. T. Reardon, A. Sancar, K. M. Giacomini and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8902–8907 CrossRef CAS PubMed.
- P. Perego, C. Caserini, L. Gatti, N. Carenini, S. Romanelli, R. Supino, D. Colangelo, I. Viano, R. Leone, S. Spinelli, G. Pezzoni, C. Manzotti, N. Farrell and F. Zunino, Mol. Pharmacol., 1999, 55, 528–534 CAS.
- A. M. Krause-Heuer, R. Grünert, S. Kühne, M. Buczkowska, N. J. Wheate, D. D. Le Pevelen, L. R. Boag, D. M. Fisher, J. Kasparkova, J. Malina, P. J. Bednarski, V. Brabec and J. R. Aldrich-Wright, J. Med. Chem., 2009, 52, 5474–5484 CrossRef CAS PubMed.
- T. Zou, C. Lok, Y. M. E. Funga and C. M. Che, Chem. Commun., 2013, 49, 5423–5425 RSC.
- A. Naik, R. Rubbiani, G. Gasser and B. Spingler, Angew. Chem., Int. Ed., 2014, 53, 6938–6941 CrossRef CAS PubMed.
- J. P. Macquet and J. L. Butour, J. Natl. Cancer Inst., 1983, 70, 899–905 CAS.
- X. Han, J. Sun, Y. Wang and Z. He, Med. Res. Rev., 2015, 35, 1268–1299 CrossRef CAS PubMed.
- J. L. Van der Veer, A. R. Peters and J. Reedijk, J. Inorg. Biochem., 1986, 26, 137–142 CrossRef CAS.
- R. M. Roat and J. Reedijk, J. Inorg. Biochem., 1993, 52, 263–274 CrossRef CAS.
- K. Lemma, J. Berglund, N. Farrell and L. I. Elding, JBIC, J. Biol. Inorg. Chem., 2000, 5, 300–306 CrossRef CAS PubMed.
- N. J. Wheate, S. Walker, G. E. Craig and R. Oun, Dalton Trans., 2010, 39, 8113–8127 RSC.
- Q. Mi, S. S. Shu, C. X. Yang, C. Gao, X. Zhang, X. Luo, C. H. Bao, X. Zhang and J. Niu, Clin. Eng. Radiat. Oncol., 2018, 7, 231–247 Search PubMed.
- M. Gordon and S. Hollander, J. Med., 1993, 24, 209–265 CAS.
- V. H. C. Bramwell, D. Crowther, S. O'Malley, R. Swindell, R. Johnson, E. H. Cooper, N. Thatcher and A. Howell, Cancer Treat. Rep., 1985, 69, 409–416 CAS.
- M. J. McKeage, F. Raynaud, J. Ward, C. Berry, D. O'Dell, L. R. Kelland, B. Murrer, P. Santabárabara, K. R. Harrap and I. R. Judson, J. Clin. Oncol., 1997, 15, 2691–2700 CrossRef CAS PubMed.
- U. Olszewski, F. Ach, E. Ulsperger, G. Baumgartner, R. Zeillinger, P. Bednarski and G. Hamilton, Met.-Based Drugs, 2009, 2009, 348916 Search PubMed.
- R. E. Cameron and A. B. Bocarsly, Inorg. Chem., 1986, 25, 2910–2913 CrossRef CAS.
- P. J. Bednarski, F. S. Mackay and P. J. Sadler, Anticancer Agents Med. Chem., 2007, 7, 75–93 CrossRef CAS PubMed.
- M. Imran, W. Ayub, I. S. Butler and Z. Rehman, Coord. Chem. Rev., 2018, 376, 405–429 CrossRef CAS.
- J. Šima, Coord. Chem. Rev., 2006, 250, 2325–2334 CrossRef.
- V. Vreeken, M. A. Siegler, B. de Bruin, J. N. H. Reek, M. Lutz and J. I. Vlugt, Angew. Chem., Int. Ed., 2015, 54, 7055–7059 CrossRef CAS PubMed.
- A. Vogler and A. Kern, Angew. Chem., Int. Ed. Engl., 1978, 17, 524–525 CrossRef.
- A. Vogler and J. Hlavatsch, Angew. Chem., Int. Ed. Engl., 1983, 22, 154–155 CrossRef.
- A. Vogler, C. Quett and H. Kunkely, Ber. Bunsen-Ges. Phys. Chem., 1988, 92, 1486–1492 CrossRef CAS.
- P. J. Bednarski, K. Korpis, A. F. Westendorf, S. Perfahl and R. Grünert, Philos. Trans. R. Soc., A, 2013, 371, 20120118 CrossRef PubMed.
- X. Wang, X. Wang, S. Jin, N. Muhammad and Z. Guo, Chem. Rev., 2019, 119, 1138–1192 CrossRef CAS PubMed.
- N. J. Farrer and P. J. Sadler, Aust. J. Chem., 2008, 61, 669–674 CrossRef CAS.
- N. A. Smith and P. J. Sadler, Philos. Trans. R. Soc., A, 2013, 371, 20120519 CrossRef PubMed.
- P. Heringiova, J. Woods, F. M. Mackay, J. Kasparkova, P. J. Sadler and V. Brabec, J. Med. Chem., 2006, 49, 7792–7798 CrossRef PubMed.
- Y. Zhao, G. M. Roberts, S. E. Greenough, N. J. Farrer, M. J. Paterson, W. H. Powell, V. G. Stavros and P. J. Sadler, Angew. Chem., Int. Ed., 2012, 51, 11263–11266 CrossRef CAS PubMed.
- K. Mitra, S. Gautam, P. Kondaiah and A. R. Chakravarty, Angew. Chem., Int. Ed., 2015, 54, 13989–13993 CrossRef CAS PubMed.
- K. Mitra, C. E. Lyons and M. C. T. Hartman, Angew. Chem., Int. Ed., 2018, 57, 10263–10267 CrossRef CAS PubMed.
- H. Shi, G. J. Clarkson and P. J. Sadler, Inorg. Chim. Acta, 2019, 489, 230–235 CrossRef CAS.
- D. Liu, J. Ma, W. Zhou, W. He and Z. Guo, Inorg. Chim. Acta, 2012, 393, 198–203 CrossRef CAS.
- K. L. Ciesienski, L. M. Hyman, D. T. Yang, K. L. Haas, M. G. Dickens, R. J. Holbrook and K. J. Franz, Eur. J. Inorg. Chem., 2010, 15, 2224–2228 CrossRef.
- A. Presa, G. Vázquez, L. A. Barrios, O. Roubeau, L. Korrodi-Gregório, R. Pérez-Tomás and P. Gamez, Inorg. Chem., 2018, 57, 4009–4022 CrossRef CAS PubMed.
- A. Presa, R. F. Brissos, A. B. Caballero, I. Borilovic, L. Korrodi-Gregório, R. Pérez-Tomás, O. Roubeau and P. Gamez, Angew. Chem., Int. Ed., 2015, 54, 4561–4565 CrossRef CAS PubMed.
- S. Monro, K. L. Colón, H. Yin, J. Roque, P. Konda, S. Gujar, R. P. Thummel, L. Lilge, C. G. Cameron and S. A. McFarland, Chem. Rev., 2019, 119, 797–828 CrossRef CAS PubMed.
- Q. Chen, Z. Huang, D. Luck, J. Beckers, P. Brun, B. C. Wilson, A. Scherz, Y. Salomon and F. W. Hetzel, Photochem. Photobiol., 2002, 76, 438–445 CrossRef CAS PubMed.
- A. N. Hidayatullah, E. Wachter, D. K. Heidary, S. Parkin and E. C. Glazer, Inorg. Chem., 2014, 53, 10030–10032 CrossRef CAS PubMed.
- L. N. Lameijer, D. Ernst, S. L. Hopkins, M. S. Meijer, S. H. C. Askes, S. E. Le Dévédec and S. Bonnet, Angew. Chem., Int. Ed., 2017, 56, 11549–11553 CrossRef CAS PubMed.
- S. L. H. Higgins and K. J. Brewer, Angew. Chem., Int. Ed., 2012, 51, 11420–11422 CrossRef CAS PubMed.
- M. H. Al-Afyouni, T. N. Rohrabaugh, J. K. F. Al-Afyouni and C. Turro, Chem. Sci., 2018, 9, 6711–6720 RSC.
- L. T. Ellis, H. M. Er and T. W. Hambley, Aust. J. Chem., 1995, 48, 793–806 CrossRef CAS.
- A. M. Pizarro, R. J. McQuitty, F. S. Mackay, Y. Zhao, J. A. Woods and P. J. Sadler, ChemMedChem, 2014, 9, 1169–1175 CrossRef CAS PubMed.
- N. A. Kratochwil, P. J. Bednarski, H. Mrozek, A. Vogler and J. K. Nagle, Anticancer Drug Des., 1996, 11, 155–171 CAS.
- N. A. Kratochwil, M. Zabel, K. J. Range and P. J. Bednarski, J. Med. Chem., 1996, 39, 2499–2507 CrossRef CAS PubMed.
- N. A. Kratochwil, J. A. Parkinson, P. J. Bednarski and P. J. Sadler, Angew. Chem., Int. Ed., 1999, 38, 1460–1463 CrossRef CAS.
- N. A. Kratochwil, Z. Guo, P. S. Murdoch, J. A. Parkinson, P. J. Bednarski and P. J. Sadler, J. Am. Chem. Soc., 1998, 120, 8253–8254 CrossRef CAS.
- N. A. Kratochwil and P. J. Bednarski, Arch. Pharm. Pharm. Med. Chem., 1999, 332, 279–285 CrossRef CAS PubMed.
- J. A. Duffy, J. Phys. C: Solid State Phys., 1980, 13, 2979–2989 CrossRef CAS.
- P. Müller, B. Schröder, J. A. Parkinson, N. A. Kratochwil, R. A. Coxall, A. Parkin, S. Parsons and P. J. Sadler, Angew. Chem., Int. Ed., 2003, 42, 335–339 CrossRef PubMed.
- P. J. Bednarski, R. Grünert, M. Zielzki, A. Wellner, F. S. Mackay and P. J. Sadler, Chem. Biol., 2006, 13, 61–67 CrossRef CAS PubMed.
- F. S. Mackay, J. A. Woods, H. Moseley, J. Ferguson, A. Dawson, S. Parsons and P. J. Sadler, Chem. – Eur. J., 2006, 12, 3155–3161 CrossRef CAS PubMed.
- M. J. Cleare and J. D. Hoeschele, Platinum Met. Rev., 1973, 17, 2–13 CAS.
- M. J. Cleare and J. D. Hoeschele, Bioinorg. Chem., 1973, 2, 187–210 CrossRef CAS.
- B. Rosenberg, L. Van Camp, E. B. Grimley and A. J. Thomson, J. Biol. Chem., 1967, 242, 1347–1352 CAS.
- N. J. Farrer, J. A. Woods, V. P. Munk, F. S. Mackay and P. J. Sadler, Chem. Res. Toxicol., 2010, 23, 413–421 Search PubMed.
- H. C. Tai, Y. Zhao, N. J. Farrer, A. E. Anastasi, G. Clarkson, P. J. Sadler and R. J. Deeth, Chem. – Eur. J., 2012, 18, 10630–10642 CrossRef CAS PubMed.
- F. S. Mackay, S. A. Moggach, A. Collins, S. Parsons and P. J. Sadler, Inorg. Chim. Acta, 2009, 362, 811–819 CrossRef CAS.
- F. S. Mackay, J. A. Woods, P. Heringová, J. Kašpárková, A. M. Pizarro, S. A. Moggach, S. Parsons, V. Brabec and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20743–20748 CrossRef CAS PubMed.
- A. F. Westendorf, J. A. Woods, K. Korpis, N. J. Farrer, L. Salassa, K. Robinson, V. Appleyard, K. Murray, R. Grünert, A. M. Thompson, P. J. Sadler and P. J. Bednarski, Mol. Cancer Ther., 2012, 11, 1894–1904 CrossRef CAS PubMed.
- A. F. Westendorf, L. Zerzankova, L. Salassa, P. J. Sadler, V. Brabec and P. J. Bednarski, J. Inorg. Biochem., 2011, 105, 652–662 CrossRef CAS PubMed.
- Y. Zhao, J. A. Woods, N. J. Farrer, K. S. Robinson, J. Pracharova, J. Kasparkova, O. Novakova, H. Li, L. Salassa, A. M. Pizarro, G. J. Clarkson, L. Song, V. Brabec and P. J. Sadler, Chem. – Eur. J., 2013, 19, 9578–9591 CrossRef CAS PubMed.
- N. J. Farrer, J. A. Woods, L. Salassa, Y. Zhao, K. S. Robinson, G. Clarkson, F. S. Mackay and P. J. Sadler, Angew. Chem., Int. Ed., 2010, 49, 8905–8908 CrossRef CAS PubMed.
- H. Shi, I. Romero-Canelón, M. Hreusova, O. Novakova, V. Venkatesh, A. Habtemariam, G. J. Clarkson, J. Song, V. Brabec and P. J. Sadler, Inorg. Chem., 2018, 57, 14409–14420 CrossRef CAS PubMed.
- F. S. Mackay, N. J. Farrer, L. Salassa, H. C. Tai, R. J. Deeth, S. A. Moggach, P. A. Wood, S. Parsons and P. J. Sadler, Dalton Trans., 2009, 2315–2325 RSC.
- Y. Nakabayashi, A. Erxleben, U. Létinois, G. Pratviel, B. Meunier, L. Holland and B. Lippert, Chem. – Eur. J., 2007, 13, 3980–3988 CrossRef CAS PubMed.
- C. Loup, A. T. Vallina, Y. Coppel, U. Létinois, Y. Nakabayashi, B. Meunier, B. Lippert and G. Pratviel, Chem. – Eur. J., 2010, 16, 11420–11431 CrossRef CAS PubMed.
- L. Cubo, A. M. Pizarro, A. Gómez Quiroga, L. Salassa, C. Navarro-Ranninger and P. J. Sadler, J. Inorg. Biochem., 2010, 104, 909–918 CrossRef CAS PubMed.
- H. Xiao, L. Yan, E. M. Dempsey, W. Song, R. Qi, W. Li, Y. Huang, X. Jing, D. Zhou, J. Ding and X. Chen, Prog. Polym. Sci., 2018, 87, 70–106 CrossRef CAS.
- D. Guo, S. Xu, Y. Huang, H. Jiang, W. Yasen, N. Wang, Y. Su, J. Qian, J. Li, C. Zhang and X. Zhu, Biomaterials, 2018, 177, 67–77 CrossRef CAS PubMed.
- A. Y. Sokolov and H. F. Schaefer III, Dalton Trans., 2011, 40, 7571–7582 RSC.
- L. Ronconi and P. J. Sadler, Chem. Commun., 2008, 2, 235–237 RSC.
- H. I. A. Phillips, L. Ronconi and P. J. Sadler, Chem. – Eur. J., 2009, 15, 1588–1596 CrossRef CAS PubMed.
- L. Salassa, H. I. A. Phillips and P. J. Sadler, Phys. Chem. Chem. Phys., 2009, 11, 10311–10316 RSC.
- L. Ronconi and P. J. Sadler, Dalton Trans., 2011, 40, 262–268 RSC.
- A. A. Shushakov, I. P. Pozdnyakov, V. P. Grivin, V. F. Plyusnin, D. B. Vasilchenko, A. V. Zadesenets, A. A. Melnikov, S. V. Chekalind and E. M. Glebov, Dalton Trans., 2017, 46, 9440–9450 RSC.
- A. F. Westendorf, A. Bodtke and P. J. Bednarski, Dalton Trans., 2011, 40, 5342–5351 RSC.
- A. F. Westendorf, Synthese und Charakterisierung von photoaktivierbaren trans-Pt(IV)-Diaziden und Evaluierung iher DNA-bindenden und antiproliferativerenden Eigenschaften an Krebszellen unter Einfluss von Licht, Dissertation, University of Greifswald, Germany, 2012 Search PubMed.
- Y. Zhao, N. J. Farrer, H. Li, J. S. Butler, R. J. McQuitty, A. Habtemariam, F. Wang and P. J. Sadler, Angew. Chem., Int. Ed., 2013, 52, 13633–13637 CrossRef CAS PubMed.
- R. R. Vernooij, T. Joshi, M. D. Horbury, B. Graham, E. I. Izgorodina, V. G. Stavros, P. J. Sadler, L. Spiccia and B. R. Wood, Chem. – Eur. J., 2018, 24, 5790–5803 CrossRef CAS PubMed.
- D. P. Bancroft, C. A. Lepre and S. J. Lippard, J. Am. Chem. Soc., 1990, 112, 6860–6871 CrossRef CAS.
- M. Crul, R. C. van Waardenburg, J. H. Beijnen and J. H. Schellens, Cancer Treat. Rev., 2002, 28, 291–303 CrossRef CAS PubMed.
- J. Kašpárková, F. S. Mackay, V. Brabec and P. J. Sadler, JBIC, J. Biol. Inorg. Chem., 2003, 8, 741–745 CrossRef PubMed.
- J. Pracharova, L. Zerzankova, J. Stepankova, O. Novakova, N. J. Farrer, P. J. Sadler, V. Brabec and J. Kasparkova, Chem. Res. Toxicol., 2012, 25, 1099–1111 Search PubMed.
- H. C. Tai, R. Brodbeck, J. Kasparkova, N. J. Farrer, V. Brabec, P. J. Sadler and R. J. Deeth, Inorg. Chem., 2012, 51, 6830–6841 CrossRef CAS PubMed.
- J. S. Butler, J. A. Woods, N. J. Farrer, M. E. Newton and P. J. Sadler, J. Am. Chem. Soc., 2012, 134, 16508–16511 CrossRef CAS PubMed.
- C. Vallotto, E. Shaili, H. Shi, J. S. Butler, C. J. Wedge, M. E. Newton and P. J. Sadler, Chem. Commun., 2018, 54, 13845–13848 RSC.
- C. A. Wootton, C. Sanchez-Cano, A. F. Lopez-Clavijo, E. Shaili, M. P. Barrow, P. J. Sadler and P. B. O'Connor, Chem. Sci., 2018, 9, 2733–2739 RSC.
- A. Holmgren and J. Lu, Biochem. Biophys. Res. Commun., 2010, 396, 120–124 CrossRef CAS PubMed.
- J. Du, Y. Wei, Y. Zhao, F. Xu, Y. Wang, W. Zheng, Q. Luo, M. Wang and F. Wang, Inorg. Chem., 2018, 57, 5575–5584 CrossRef CAS PubMed.
- M. D. Hall and T. W. Hambley, Coord. Chem. Rev., 2002, 232, 49–267 CrossRef CAS.
- V. Venkatesh, C. J. Wedge, I. Romero-Canelón, A. Habtemariama and P. J. Sadler, Dalton Trans., 2016, 45, 13034–13037 RSC.
- L. Ning, D. Y. Greenblatt, M. Kunnimalaiyaan and H. Chen, Oncologist, 2008, 13, 98–104 CrossRef CAS PubMed.
- J. Kasparkova, H. Kostrhunova, O. Novakova, R. Křikavová, J. Vančo, Z. Trávníček and V. Brabec, Angew. Chem., Int. Ed., 2015, 54, 14478–14482 CrossRef CAS PubMed.
- S. Banerjee and A. R. Chakravarty, Acc. Chem. Res., 2015, 48, 2075–2083 CrossRef CAS PubMed.
- S. He, Y. Qi, G. Kuang, D. Zhou, J. Li, Z. Xie, X. Chen, X. Jing and Y. Huang, Biomacromolecules, 2016, 17, 2120–2127 CrossRef CAS PubMed.
- S. Xu, X. Zhu, C. Zhang, W. Huang, Y. Zhou and D. Yan, Nat. Commun., 2018, 9, 2053 CrossRef PubMed.
- S. S. Kesharwania, S. Kaurb, H. Tummala and A. T. Sangamwar, Colloids Surf., B, 2019, 173, 581–590 CrossRef PubMed.
- Y. Min, J. Li, F. Liu, E. K. L. Yeow and B. Xing, Angew. Chem., Int. Ed., 2014, 53, 1012–1016 CrossRef CAS PubMed.
- X. Li, J. Mu, F. Liu, E. W. P. Tan, B. Khezri, R. D. Webster, E. K. L. Yeow and B. Xing, Bioconjugate Chem., 2015, 26, 955–961 CrossRef CAS PubMed.
- Y. Dai, H. Xiao, J. Liu, Q. Yuan, P. Ma, D. Yang, C. Li, Z. Cheng, Z. Hou, P. Yang and J. Lin, J. Am. Chem. Soc., 2013, 135, 18920–18929 CrossRef CAS PubMed.
- M. Friedlander, P. C. Brooks, R. W. Shaffer, C. M. Kincaid, J. A. Varner and D. A. Cheresh, Science, 1995, 270, 1500–1502 CrossRef CAS PubMed.
- J. S. Desgrosellier and D. A. Cheresh, Nat. Rev. Cancer, 2010, 10, 9–22 CrossRef CAS PubMed.
- A. Gandioso, E. Shaili, A. Massaguer, G. Artigas, A. González-Cantó, J. A. Woods, P. J. Sadler and V. Marchán, Chem. Commun., 2015, 51, 9169–9172 RSC.
- E. Shaili, M. Fernández-Giménez, S. Rodríguez-Astor, A. Gandioso, L. Sandín, C. García-Vélez, A. Massaguer, G. J. Clarkson, J. A. Woods, P. J. Sadler and V. Marchán, Chem. – Eur. J., 2015, 21, 18474–18486 CrossRef CAS PubMed.
- H. Maeda, J. Controlled Release, 2012, 164, 138–144 CrossRef CAS PubMed.
- H. Wei, R. Zhuo and X. Zhang, Prog. Polym. Sci., 2013, 38, 503–535 CrossRef CAS.
- X. Yang, H. Xiang, L. An, S. Yang and J. Liu, New J. Chem., 2015, 39, 800–804 RSC.
- Y. Zhao, Macromolecules, 2012, 45, 3647–3657 CrossRef CAS.
- H. Xiao, G. T. Noble, J. F. Stefanick, R. Qi, T. Kiziltepe, X. Jing and B. Bilgicer, J. Controlled Release, 2014, 173, 11–17 CrossRef CAS PubMed.
- H. Song, W. Li, R. Qi, L. Yan, X. Jing, M. Zheng and H. Xiao, Chem. Commun., 2015, 51, 11493–11495 RSC.
- H. Song, X. Kang, J. Sun, X. Jing, Z. Wang, L. Yan, R. Qi and M. Zheng, Chem. Commun., 2016, 52, 2281–2283 RSC.
- D. Zhou, J. Guo, G. B. Kim, J. Li, X. Chen, J. Yang and Y. Huang, Adv. Healthcare Mater., 2016, 5, 2493–2499 CrossRef CAS PubMed.
- S. He, C. Li, Q. Zhang, J. Ding, X. Liang, X. Chen, H. Xiao, X. Chen, D. Zhou and Y. Huang, ACS Nano, 2018, 12, 7272–7281 CrossRef CAS PubMed.
- G. M. Peters, L. P. Skala, T. N. Plank, B. J. Hyman, G. N. M. Reddy, A. Marsh, S. P. Brown and J. T. Davis, J. Am. Chem. Soc., 2014, 136, 12596–12599 CrossRef CAS PubMed.
- G. M. Peters, L. P. Skala, T. N. Plank, H. Oh, G. N. M. Reddy, A. Marsh, S. P. Brown, S. R. Raghavan and J. T. Davis, J. Am. Chem. Soc., 2015, 137, 5819–5827 CrossRef CAS PubMed.
- T. N. Plank and J. T. Davis, Chem. Commun., 2016, 52, 5037–5040 RSC.
- V. Venkatesh, N. K. Mishra, I. Romero-Canelón, R. R. Vernooij, H. Shi, J. P. C. Coverdale, A. Habtemariam, S. Verma and P. J. Sadler, J. Am. Chem. Soc., 2017, 139, 5656–5565 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2019 |