Open Access Article
Open Access ArticleHyperfine coupling and slow magnetic relaxation in isotopically enriched DyIII mononuclear single-molecule magnets†
Jessica
Flores Gonzalez
,
Fabrice
Pointillart
 * and
Olivier
Cador
* and
Olivier
Cador
 *
*
Univ Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes) - UMR 6226, F-35000 Rennes, France. E-mail: olivier.cador@univ-rennes1.fr
First published on 19th March 2019
Abstract
The four main stable isotopes of the [ADy(tta)3(L)]·C6H14 (ADy with A = 161–164) Single-Molecule Magnet (SMM) (tta− = 2-thenoyltrifluoroacetylacetonate and L = 2-{[2-methylpyridiyl]-4,5-[4,5-bis(propylthio)-tetrathiafulvalenyl]-1H-benzimidazol-2-yl}pyridine) have been magnetically investigated and structurally characterized by single crystal X-ray diffraction. The two nuclear spin-free 162/164Dy behave the same from a dynamic magnetic point of view and with slower magnetic relaxation than the two pure magnetically active isotopically substituted 161/163Dy. In addition, this paper demonstrates that 161Dy and 163Dy relax differently even if they both carry I = 5/2 nuclear spin. After the release of the dipolar interactions through magnetic dilution, ([161Dy0.05Y0.95(tta)3(L)]·C6H14) (161Dy@Y) relaxes four times slower than 163Dy@Y due to different hyperfine coupling constants.
Introduction
The possibility to observe a magnetic memory at the single-ion level for a lanthanide ion was demonstrated in 2003 for a [TbPc2] (Pc = phthalocyaninato) complex.1,2 Since that discovery, lanthanide-based mononuclear Single-Molecule Magnets (SMMs) have been designed for their potential applications in high-density data storage3 and spintronic devices.4On one hand, the integration of lanthanide SMMs in devices for data storage is limited due to the low-temperature range at which such molecular systems highlight magnetic memory. To overcome such a limitation, few strategies have been explored: (i) a molecular engineering strategy to induce an adequate symmetry and crystal field around the lanthanide ion leading to an optimal crystal splitting of the ground multiplet state. Such a strategy has recently driven chemists to design a hexa-tert-butyldysprosocenium complex with an exceptional magnetic axiality and a blocking temperature of 60 K;5,6,7–10 (ii) the minimization of the magnetic moment perturbation by magnetic dilution by adding the complex to a frozen solution11 or doping it with a diamagnetic isomorphous matrix12 which leads to the isolation of the electronic spin carriers; and (iii) the minimization of the hyperfine interactions by isotopic enrichment in nuclear spin-free lanthanides which leads to a diminution of the Quantum Tunnelling of Magnetization (QTM) efficiency.13,14
On the other hand, lanthanide SMMs could be suitable to perform nuclear spin qubits/qudits4,15–18 due to the several DiVincenzo characteristics19,20 already observed for such systems. In other words, two nuclear isomers of a lanthanide SMM could be used to enhance the magnetic performance for data storage (nuclear spin-free isomer) while the pure nuclear spin isomer could be used as a multilevel nuclear spin qubit (qudit) because of the nuclear-spin-driven QTM events.21 Nevertheless the DyIII ion is composed of seven isotopes with nuclear spin values of 0 and 5/2. The four main 161Dy, 162Dy, 163Dy and 164Dy isotopes represent 97.5% of the isotopic mixture but until now only two of them have been studied on the same system.13,14,21
In this paper, we propose to go a step further in studying four stable isotopically enriched dysprosium complexes ([ADy(tta)3(L)]·C6H14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 where tta− = 2-thenoyltrifluoroacetylacetonate, L = 2-{[2-methylpyridiyl]-4,5-[4,5-bis(propylthio)-tetrathiafulvalenyl]-1H-benzimidazol-2-yl}pyridine) and A = 161, 162, 163 and 164 (abbreviated as ADy here after). It is worth noting that 161Dy and 164Dy have been previously studied by some of us.13 The aim of this study was to pursue the investigations on an identical system for which all the synthetic steps are under control. The two 161Dy and 163Dy isotopes possess the same nuclear spin value (I = 5/2) but not the same nuclear magnetic moment while the 162Dy and 164Dy isotopes possess no nuclear spins. Magnetic dilution of the dysprosium complexes in an isomorphous diamagnetic matrix based on yttrium (ADy@Y) has been systematically performed to cancel dipolar interactions in the condensed phase.
22 where tta− = 2-thenoyltrifluoroacetylacetonate, L = 2-{[2-methylpyridiyl]-4,5-[4,5-bis(propylthio)-tetrathiafulvalenyl]-1H-benzimidazol-2-yl}pyridine) and A = 161, 162, 163 and 164 (abbreviated as ADy here after). It is worth noting that 161Dy and 164Dy have been previously studied by some of us.13 The aim of this study was to pursue the investigations on an identical system for which all the synthetic steps are under control. The two 161Dy and 163Dy isotopes possess the same nuclear spin value (I = 5/2) but not the same nuclear magnetic moment while the 162Dy and 164Dy isotopes possess no nuclear spins. Magnetic dilution of the dysprosium complexes in an isomorphous diamagnetic matrix based on yttrium (ADy@Y) has been systematically performed to cancel dipolar interactions in the condensed phase.
Materials and methods
The elemental analyses of the compounds were performed at the Centre Régional de Mesures Physiques de l'Ouest, Rennes. The Dy/Y ratio of the compounds 162Dy@Y and 163Dy@Y was determined using SEM (Scanning Electron Microscopy). All observations and measurements were carried out with a JEOL JSM 6400 scanning electron microscope (JEOL Ltd, Tokyo, Japan) with an EDS (Energy Dispersive Spectrometry) analysis system (Oxford Link INCA). The voltage was kept at 9 kV, and the samples were mounted on carbon stubs and coated for 5 min with a gold/palladium alloy using a sputter coater (Jeol JFC 1100). This analysis has been performed by the “Centre de Microscopie Electronique à Balayage et microAnalyse (CMEBA)” from the University of Rennes 1 (France). The dc magnetic susceptibility measurements were performed on solid polycrystalline samples with a Quantum Design MPMS-XL SQUID magnetometer between 2 and 300 K in an applied magnetic field of 200 Oe for temperatures between 2 and 20 K, 2 kOe between 20 and 80 K and 10 kOe above 80 K. Making measurements at various applied fields allows us to verify that the samples are free from ferromagnetic impurities and also avoid saturation phenomena at low temperature. These measurements were all corrected for the diamagnetic contribution as calculated with Pascal's constants.Experimental
All solvents were dried using standard procedures. 4,5-Bis(propylthio)-tetrathiafulvalene-2-(2-pyridyl)benzimidazole-methyl-2-pyridine ligand (L) was synthesized following a published procedure.23 The isotopically enriched Dy2O3 oxides are commercially available from Eurisotop company. All other reagents were purchased from Aldrich Co. Ltd and were used without further purification.Isotopically enriched Dy(tta)3·2H2O compounds are prepared according to the following modified literature procedure:24 on one side, 47.1 mg (0.126 mmol) of Dy2O3 enriched at 94.4% in 162DyIII or at 95.0% in 163DyIII were placed in the presence of 130 μL of concentrated HCl. After 30 min of stirring at 50 °C, the resulting solution of 162/163DyCl3·6H2O was diluted with 1 mL of water. On the other side, 224 mg of Htta (1.010 mmol) were dissolved in 180 mL of water at 60 °C under intense stirring. The pH of the solution was adjusted to 6–6.5 with NH4OH. To the obtained solution was added an aqueous solution of isotopically enriched DyCl3·6H2O (0.252 mmol). The pH of the solution was adjusted to 7–7.5 during the stirring and then flocculation of Dy(tta)3·2H2O took place and was filtered. Yield 162Dy(tta)3·2H2O: 115 mg (53%). Yield 163Dy(tta)3·2H2O: 110 mg (51%).
[162/163Dy(tta)3(L)]·C6H14 (162Dy and 163Dy): a CH2Cl2 solution of L (24.4 mg, 0.04 mmol) and 162/163Dy(tta)3·2H2O (34.5 mg, 0.04 mmol) is stirred for 1 hour. n-Hexane is layered to the solution and red prismatic single crystals are obtained after two weeks of diffusion and evaporation at room temperature. Yield 162Dy: 47.7 mg (83%). Yield 163Dy: 50.0 mg (87%). Anal. calcd (%) for C52H38DyF9N4O6S9: C 43.47, H 2.65, N 3.90; found 162Dy: C 43.61, H 2.72, N 3.79. found 163Dy: C 43.66, H 2.77, N 3.83.
[162/163Dy0.05Y0.95(tta)3(L)]·C6H14 (162/163Dy@Y): a CH2Cl2 solution (15 mL) of L (61.0 mg, 0.1 mmol) is added to a CH2Cl2 solution (10 mL) containing 162/163Dy(tta)3·2H2O (3.4 mg, 0.004 mmol) and Y(tta)3·2H2O (75.7 mg, 0.096 mmol) and stirred for 1 hour. n-Hexane is layered to the solution and red prismatic single crystals are obtained after two weeks of diffusion and evaporation at room temperature. Yield 162Dy@Y: 47.7 mg (80%). Yield 163Dy@Y: 50.0 mg (85%). C52H38Dy0.05Y0.95F9N4O6S9: C 45.69, H 2.78, N 4.10; found 162Dy: C 45.60, H 2.82, N 3.99; found 163Dy: C 45.66, H 2.87, N 4.03.
Results and discussion
Structural description
The four compounds 162Dy, 163Dy, 162Dy@Y and 163Dy@Y are isostructural (Table S1, Fig. S1–S4†). The ratio Dy/Y (0.05/0.95) for the two doped compounds [162Dy0.05Y0.95(tta)3(L)]·C6H14 (162Dy@Y) and [163Dy0.05Y0.95(tta)3(L)]·C6H14 (163Dy@Y) has been determined by EDS analysis and then it is used to refine the X-ray structure on single crystals. The X-ray structures for the natural isotopes, 161Dy and 164Dy, have been studied in our previous work.13,22 Thus a single and rapid structural description is given below to understand the structural characteristic of the system.The X-ray structures consist of a mononuclear complex of DyIII which lies in a N2O6 coordination environment with a square antiprism symmetry. Three tta− anions and the ligand L provide the six oxygen and two nitrogen atoms, respectively (Fig. 1). As expected, the Dy–N bond length is longer than the Dy–O ones due to the oxophilic character of the lanthanide. The central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond length and the non-planar TTF core confirm the neutrality of L. The crystal packing highlighted the formation of “head-to-tail” dimers of complexes (Fig. S5†).
C bond length and the non-planar TTF core confirm the neutrality of L. The crystal packing highlighted the formation of “head-to-tail” dimers of complexes (Fig. S5†).
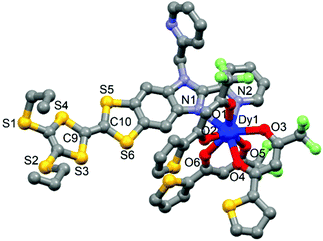 | ||
| Fig. 1 Molecular structure of 163Dy. Hydrogen atoms and CH2Cl2 molecule of crystallization are omitted for clarity. | ||
Static magnetic properties
The thermal dependence of the magnetic susceptibility and the first magnetization at 2 K are measured for the 162Dy and 163Dy samples and for 162Dy@Y and 163Dy@Y (Fig. S6 and S7†). The χMT vs. T and M vs. H curves are identical for the two isotopes because the static magnetic properties are independent of the nuclear magnetic moment. The experimental values are in agreement with the expected values for a single DyIII ion.25Dynamic magnetic properties
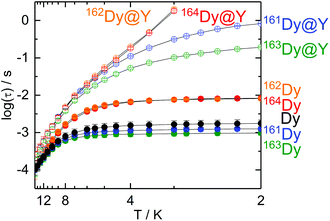
The dynamic magnetic properties of the two isotopologues 162Dy (I = 0) and 163Dy (I = 5/2) are determined by measuring the frequency dependences of the magnetic susceptibility (Fig. 2, S9 and S10†). Both isotopologues display a frequency dependence for the out-of-phase component, χM′′, of the ac susceptibility in a zero external dc field, a sign of slow magnetic relaxation. As previously observed for 161Dy and 164Dy, the maximum on the χM′′ vs. ν curve (where ν is the frequency of the oscillating field) occurs at a lower frequency for the nuclear spin-free isotope (16 Hz) than for the isotope with I = 5/2 (160 Hz).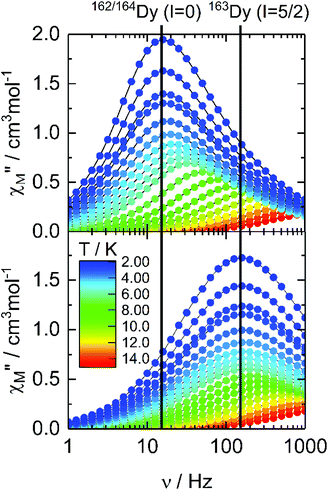 | ||
| Fig. 2 Frequency dependences of χM′′ of 162Dy and 163Dy in a zero magnetic field in the temperature range of 2–15 K. | ||
The relaxation times (τ) for both isotopologues have been extracted with an extended Debye model (see the ESI†). Their temperature dependences follow a modified Arrhenius law (τ−1 = τ0−1 exp(Δ/T) + τTI−1) (where τ0 and τTI are, respectively, the intrinsic and temperature-independent relaxation times and Δ is the energy barrier) (Fig. 3, Tables 1, S2 and S3†). Both Δ and τ0 remain almost identical for 162Dy and 163Dy, suggesting that the thermally activated regime is not affected by the nucleus and thus by the hyperfine interactions (Table 1, Fig. S11 and S12†), while the thermally independent regime (QTM) is strongly affected by the isotopic enrichment with the cancellation of the nuclear spin of dysprosium (Fig. 3). Even if the QTM is reduced, it is still operative at a low temperature. To cancel this, an optimal magnetic field of 1000 Oe is applied (Fig. S13–S16†), leading to a drastic slowing down of the magnetic relaxation. Nevertheless, such an intense magnetic field also cancels the isotopic effect. In fact the two Arrhenius plots for both 162Dy and 163Dy are superimposed (Fig. S17†). Fig. 3 provides an easy comparison between all isotopologues. As expected, the two nuclear spin-free isotopes 162Dy and 164Dy behave the same whereas the two isotopes 161Dy and 163Dy (I = 5/2) display faster magnetic relaxation. In the QTM regime, a significant difference exists between 161Dy and 163Dy. Fig. 4 shows the χM′′ vs. ν curves for the two 161Dy and 163Dy with respective maxima centred at 106 Hz and 160 Hz, respectively. 163Dy relaxes slightly faster than 161Dy. This can be compared with the very different (of opposite sign) hyperfine coupling constants of 161Dy and 163Dy.26 Furthermore, the hyperfine interactions are expected to be stronger for the isotope 163Dy than for 161Dy,27 leading to a more efficient QTM for the former as experimentally observed (Fig. 3 and Table 1). The effect of the nuclear spin value and, a fortiori, the effect of the hyperfine constant value are very sensitive to any magnetic field (external or internal) because the hyperfine interaction, of the order of a few thousandths of wavenumber, is easily destroyed by the dipolar magnetic field generated by intermolecular interactions. In order to evaluate the exact effect of the hyperfine coupling constants for the two 161Dy and 163Dy isotopes, the internal magnetic field has been released in the doped samples [161Dy0.03Y0.97(tta)3(L)]·C6H14 (161Dy@Y, the data come from our previous work13) and [163Dy0.05Y0.95(tta)3(L)]·C6H14163Dy@Y. The frequency dependences, in the zero external field, of the two doped isotopologues are depicted in Fig. 5 and S18–S21.† The cancelling of the internal magnetic field has two consequences on the magnetic properties of the doped samples: (1) the maxima of the out-of-phase component of the magnetic susceptibility are shifted to lower frequencies for both 161Dy@Y (0.2 Hz) and 163Dy@Y (0.8 Hz) with respect to the condensed phase (Fig. 3 and Tables 1, S7†). This is even true at the lowest temperatures where the relaxation time becomes almost 1000 times slower than that in the condensed phase. (2) The difference between the two doped isotopologues (161Dy@Y and 163Dy@Y) is much more pronounced, with, at 2 K, the relaxation time of 161Dy@Y being four times slower than that of 163Dy@Y. Considering that the magnetic entities in 161Dy@Y and 163Dy@Y are perfectly isolated systems, the difference observed is entirely due to hyperfine coupling. As expected, the relaxation tends to become thermally independent at low temperature. Therefore, a hyperfine coupling effect on the magnetic relaxation of a molecule can only be probed in diluted systems. Of course, the release of the hyperfine coupling in nuclear spin-free systems such as 162Dy@Y and 164Dy@Y produces slower relaxation (Fig. 3 and Table S6†) which never ends in a thermally independent regime at low temperature. At this stage, relaxation is governed by Orbach or Raman processes or a combination of these two processes.
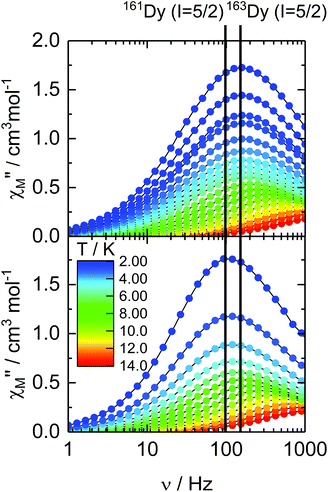 | ||
| Fig. 4 Frequency dependences of χM′′ of 161Dy and 163Dy in a zero magnetic field in the temperature range of 2–14 K. | ||
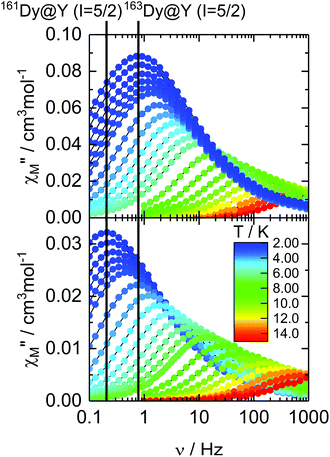 | ||
| Fig. 5 Frequency dependences of χM′′ of 161Dy@Y and 163Dy@Y in a zero magnetic field in the temperature range of 2–15 K. | ||
| Dy | 161 Dy | 162 Dy | 163 Dy | 164 Dy | |
|---|---|---|---|---|---|
| I = 5/2 | I = 0 | I = 5/2 | I = 0 | ||
| Δ/K | 43 | 45 | 44 | 46 | 43 |
| τ 0/s | 6.7(1) × 10−6 | 6.0(8) × 10−6 | 7.8(1) × 10−6 | 5.6(7) × 10−6 | 7.7(9) × 10−6 |
| τ TI/sa | 1.8(1) × 10−3 | 1.3(1) × 10−3 | 8.1(1) × 10−3 | 1.0(1) × 10−3 | 8.3(1) × 10−3 |
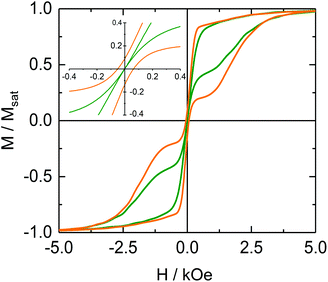
The hysteresis loops for 161Dy@Y and 163Dy@Y were compared and classical butterfly shaped hysteresis loops for the DyIII mononuclear SMM are observed but no significant differences are observed between the two isotopes (Fig. S22†), whereas the hysteresis loops of the doped compounds with the fastest (163Dy@Y) and the slowest (162Dy@Y or 164Dy@Y) relaxation of magnetization show significant differences with a clear magnetic bistability at a zero-magnetic field for the nuclear spin-free 162Dy@Y (Fig. 6). In contrast, 163Dy@Y is closed at zero field in agreement with the relaxation measurements.
Conclusions
The two 162 and 163 isotopes of the DyIII mononuclear Single-Molecule Magnet [Dy(tta)3(L)]·C6H14 were investigated and then compared with the two 161 and 164 isotopes previously studied by some of us. The nuclear spin-free isotope (162Dy) displayed a slower magnetic relaxation in the thermally independent regime compared to the isotope 163Dy (I = 5/2) due to the cancelling of the hyperfine interactions coming from the metal centre. As expected, the two nuclear spin-free 162Dy and 164Dy behaved as the same while a significant difference of the relaxation time of magnetization was observed between the two 161Dy and 163Dy isotopic isomers (I = 5/2). At 2 K, the 163Dy isotope relaxed 50% faster than 161Dy because of the greater hyperfine interaction constants for the former compared to the latter. Finally after removing the dipolar magnetic interactions by doping, the effect of the hyperfine interaction constants on magnetic relaxation is directly observed with 163Dy@Y which relaxed 300% faster than 161Dy@Y.Future DyIII isotopic enrichment in which DyIII is in a nuclear spin-free environment is under investigation in order to determine the effect of the hyperfine coupling arising from the first neighbouring atoms and the effect of the oblate versus prolate electronic distribution of the lanthanide ion.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the CNRS, Université de Rennes and the European Research Council through the ERC-CoG 725184 MULTIPROSMM (project no. 725184).Notes and references
- R. Giraud, W. Wernsdorfer, A. M. Tkachuk, D. Mailly and B. Barbara, Phys. Rev. Lett., 2001, 87, 057203 CrossRef CAS PubMed.
- N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694–8695 CrossRef CAS PubMed.
- D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford University Press, 2006 Search PubMed.
- S. Thiele, F. Balestro, R. Ballou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Science, 2014, 344, 1135–1138 CrossRef CAS PubMed.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Angew. Chem., Int. Ed., 2017, 56, 11445–11449 CrossRef CAS PubMed.
- C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439–442 CrossRef CAS PubMed.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, eaav0652 Search PubMed.
- L. Ungur, S.-Y. Lin, J. Tang and L. F. Chibotaru, Chem. Soc. Rev., 2014, 43, 6894–6905 RSC.
- Z. Zhu, M. Guo, X.-L. Li and J. Tang, Coord. Chem. Rev., 2019, 378, 350–364 CrossRef CAS.
- P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728–1763 CrossRef CAS.
- G. Cosquer, F. Pointillart, S. Golhen, O. Cador and L. Ouahab, Chem. – Eur. J., 2013, 19, 7895–7903 CrossRef CAS PubMed.
- F. Habib, P. H. Lin, J. Long, I. Korobkov, W. Wernsdorfer and M. Murugesu, J. Am. Chem. Soc., 2011, 133, 8830–8833 CrossRef CAS PubMed.
- F. Pointillart, K. Bernot, S. Golhen, B. Le Guennic, T. Guizouarn, L. Ouahab and O. Cador, Angew. Chem., Int. Ed., 2015, 54, 1504–1507 CrossRef CAS PubMed.
- Y. Kishi, F. Pointillart, B. Lefeuvre, F. Riobé, B. Le Guennic, S. Golhen, O. Cador, O. Maury, H. Fujiwara and L. Ouahab, Chem. Commun., 2017, 53, 3575–3578 RSC.
- S. Thiele, R. Vincent, M. Holzmann, S. Klyatskaya, M. Ruben, F. Balestro and W. Wernsdorfer, Phys. Rev. Lett., 2013, 111, 037203 CrossRef CAS PubMed.
- R. Vincent, Nature, 2012, 488, 357–360 CrossRef CAS PubMed.
- D. P. O'Leary, G. K. Brennen and S. S. Bullock, Phys. Rev. A, 2006, 74, 032334 CrossRef.
- M. Leuenberger and D. Loss, Nature, 2001, 410, 789–793 CrossRef CAS PubMed.
- K. S. Pedersen, A.-M. Ariciu, S. McAdams, H. Weihe, J. Bendix, F. Tuna and S. Piligkos, J. Am. Chem. Soc., 2016, 138, 5801–5804 CrossRef CAS PubMed.
- D. Aguilà, L. A. Barrios, V. Velasco, O. Roubeau, A. Repollés, P. J. Alonso, J. Sesé, S. J. Teat, F. Luis and G. Aromí, J. Am. Chem. Soc., 2014, 136, 14215–14222 CrossRef PubMed.
- E. Moreno-Pineda, M. Damjanović, O. Fuhr, W. Wernsdorfer and M. Ruben, Angew. Chem., Int. Ed., 2017, 56, 9915–9919 CrossRef CAS PubMed.
- T. T. da Cunha, J. Jung, M.-E. Boulon, G. Campo, F. Pointillart, C. L. M. Pereira, B. Le Guennic, O. Cador, K. Bernot, F. Pineider, S. Golhen and L. Ouahab, J. Am. Chem. Soc., 2013, 135, 16332–16335 CrossRef CAS PubMed.
- G. Cosquer, F. Pointillart, J. Jung, B. Le Guennic, S. Golhen, O. Cador, Y. Guyot, A. Brenier, O. Maury and L. Ouahab, Eur. J. Inorg. Chem., 2014, 2014, 69–82 CrossRef CAS.
- A. I. Voloshin, N. Shavaleev and V. P. Kazakov, J. Lumin., 2000, 91, 49–58 CrossRef CAS.
- O. Kahn, Molecular magnetism, VCH, 1993 Search PubMed.
- W. Ebenhöh, V. J. Ehlers and J. Ferch, Z. Phys., 1967, 200, 84–92 CrossRef.
- W. J. Childs, Phys. Rev. A, 1970, 2, 1692–1701 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qi01209a |
| This journal is © the Partner Organisations 2019 |