Open Access Article
Open Access ArticleLight, temperature, and pH control of aqueous azopyridine-terminated poly(N-isopropylacrylamide) solutions†
Hao
Ren
a,
Xing-Ping
Qiu
b,
Yan
Shi
 c,
Peng
Yang
c,
Peng
Yang
 a and
Françoise M.
Winnik
a and
Françoise M.
Winnik
 *de
*de
aKey Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China
bDepartment of Chemistry, University of Montreal, CP 6128 Succursale Centre Ville, Montreal, QC H3C 3J7, Canada
cSchool of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China
dLaboratory of Polymer Chemistry, Department of Chemistry, PB 55, University of Helsinki, Helsinki FI00140, Finland. E-mail: francoise.winnik@helsinki.fi
eInternational Center for Materials Nanoarchitectonics, National Institute for Material Science, 1-1 Namiki, Tsukuba 305-0044, Japan
First published on 15th August 2019
Abstract
Azopyridines (AzPy) act as light-sensitive groups that undergo reversible cis–trans isomerization upon UV irradiation, as hydrogen-bond acceptors, and as ionizable moieties. The kinetics of the thermal cis- to trans-AzPy deactivation are slow except when the Py nitrogen is H-bonded or cationic. The properties of AzPy were used here to control the phase transition of aqueous solutions of α-azopyridine-ω-n-dodecyl-poly(N-isopropylacrylamides) (C12-PN-AzPy) with the molar mass (Mn) ranging from 5800 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1. The C12-PN-AzPy polymers form cationic star-micelles in solutions of pH 3 and flower-micelles in neutral and basic solutions. This diversity of micelle morphology underlies the temperature-, pH- and UV-irradiation-driven phase transition of aqueous C12-PN-AzPy solutions as demonstrated by turbidimetry, 1H NMR spectroscopy, and microcalorimetry. Unlike azobenzene, the commonly used photoresponsive moiety to actuate amphiphilic polymers, AzPy can affect the thermoresponsive behavior of polymers in response to three orthogonal triggers: pH, through changes in ionization; light, via trans–cis photoisomerization; and time, from hours to a few ms, via the kinetics of the dark cis–trans relaxation. The study leads the way to responsive sensors or actuators in the form of aqueous fluids, hydrogels, or films by the application of light and changes of temperature and pH in permutable sequences.
700 g mol−1. The C12-PN-AzPy polymers form cationic star-micelles in solutions of pH 3 and flower-micelles in neutral and basic solutions. This diversity of micelle morphology underlies the temperature-, pH- and UV-irradiation-driven phase transition of aqueous C12-PN-AzPy solutions as demonstrated by turbidimetry, 1H NMR spectroscopy, and microcalorimetry. Unlike azobenzene, the commonly used photoresponsive moiety to actuate amphiphilic polymers, AzPy can affect the thermoresponsive behavior of polymers in response to three orthogonal triggers: pH, through changes in ionization; light, via trans–cis photoisomerization; and time, from hours to a few ms, via the kinetics of the dark cis–trans relaxation. The study leads the way to responsive sensors or actuators in the form of aqueous fluids, hydrogels, or films by the application of light and changes of temperature and pH in permutable sequences.
Introduction
Aromatic azo compounds form an important class of dyes and pigments used for centuries as brilliant dyestuffs for textiles, paints, and many other applications.1 In the 1970s, polymers containing azobenzenes, commonly referred to as “azo-polymers”, caught the attention of photochemists and polymer scientists alike, driven by the pioneering work of Neckers among others.2 Major advances within the field of azo-polymers occurred with the emergence of the concept of azobenzenes as photosensitive “triggers” of macroscopic changes of the properties of materials and solutions, such as phase changes,3 and photo-induced motion and deformation of polymer films.4 In most cases, the photo-response is linked to the trans-to-cis photoisomerization of azobenzene chromophores attached to the polymeric structures. UV-irradiation of azobenzene causes the conversion of the trans isomer to the less stable cis isomer, which in the dark slowly relaxes to the trans form. The trans-to-cis photoisomerization of azobenzene changes the photophysical properties of the chromophore, as detected by steady-state and transient UV-Vis absorbance spectroscopy, both of which are well documented.5–7 Other physical properties of the azobenzene moiety are affected as well, most notably its dipole moment: it increases or decreases upon photoisomerization, depending on the type and position of phenyl ring substituents.8,9 Thus, photoisomerization of azobenzenes linked to a thermosensitive amphiphilic polymer affects the phase transition temperature of the polymer in water as a consequence of the different hydrophilicity of the cis and trans isomers.10,11For practical applications that require fast reversibility, it is advantageous to use azobenzenes that exhibit an extremely fast thermal cis to trans relaxation, so that a single UV light beam can induce continuous trans ↔ cis isomerizations.12 The rate of the dark cis to trans isomerization of azobenzene can be enhanced significantly through manipulations of the π electron distribution, such as para-substitution of the phenyl rings13 or the replacement of one phenyl ring of azobenzene by a pyridine ring.14,15 The dark cis–trans isomerization of azopyridine (AzPy) in the neutral form is nearly as slow as that of azobenzene. It is extremely fast (relaxation time ∼1 ms or less) for the quaternized azopyridinium (AzPy+) group. The formation of hydrogen bonds between the AzPy nitrogen and H-bond donors also greatly accelerates the thermal cis–trans isomerization.
Azopyridine-substituted water-soluble polymers are amphiphilic. They tend to self-assemble in water, adopting various morphologies as a function of their chemical composition and their environment, in particular the solution pH and the presence of H-bond acceptors.16–18 This phenomenon is exemplified in a recent study of aqueous solutions of poly(N-isopropylacrylamides) bearing an AzPy on one chain end and an n-dodecyl group on the other end (C12-PN-AzPy) (Scheme 1a).19 In neutral or basic water, they form “flower-like” core–shell micelles with a core of n-dodecyl chains and AzPy groups surrounded by a PNIPAM corona. In solutions of pH 3, the micelle morphology changes as a result of the protonation of the AzPy group. C12-PN-AzPyH+ polymers form star micelles consisting of a hydrophobic core made solely of assembled n-dodecyl groups, surrounded by extended PNIPAM chains terminated with the cationic azopyridinium group.
 | ||
| Scheme 1 (a) Chemical structure of the polymers investigated in this study; (b) schematic representation of the azopyridine end group of C12-PN-AzPy in solutions of pH 3, 7 and 10.19 | ||
Aqueous solutions of PNIPAM and its derivatives undergo a reversible phase transition upon heating past a temperature of ∼32 °C that corresponds to the coil-to-globule collapse of the polymer chains and subsequent aggregation of the dehydrated PNIPAM globules into larger mesoglobules.20–22 This property is exploited commonly to actuate temperature sensitive materials. The phase transition temperature of PNIPAM derivatives in water can be adjusted over a broad range of temperatures by linking to PNIPAM hydrophilic/hydrophobic comonomers22–24 or end groups.25,26 The introduction of stimuli sensitive components responsive to stimuli, such as light,27 pH,28,29 salinity, redox conditions,30,31 or CO2,32 expands even wider the range of possible applications of PNIPAM containing polymers. End-modified PNIPAM derivatives are particularly useful as they can drive large changes of the physical or chemical properties of the materials through minor changes of the polymer chemical composition.33
Generally, non-reactive chain ends do not affect the phase transition temperature of PNIPAM of Mn ≥ 50 kDa. In the case of shorter PNIPAM chains, as a rule, hydrophilic end groups increase the solution phase transition temperature and hydrophobic end groups decrease it.34 These effects result from changes in the hydrophilic/lipophilic balance of the modified polymer chains. Charged end groups, such as carboxylates35,36 and quaternary ammonium salts.37,38 tend to prevent the aggregation of the dehydrated PNIPAM globules through electrostatic stabilization. If the size of carboxylated PNIPAM globules is sufficiently small, compared to the wavelength of light, the solution remains clear at temperatures higher than the phase transition temperature.
We report here a study by microcalorimetry, turbidimetry, and NMR spectroscopy of the phase transition of aqueous C12-PN-AzPy solutions as a function of changes in temperature and solution pH, and upon UV-light irradiation. The study demonstrates also that through their ionizability and pH-/H-bond-dependent dark relaxation kinetics, azopyridine groups are simple, versatile, and powerful tools to modulate the temperature-induced phase transition of amphiphilic polymers, such as PNIPAM upon the application of two or three orthogonal parameters, a key outcome of this investigation.
Experimental section
Materials
Water was deionized using a Millipore Milli-Q system. The C12-PN-AzPy samples were prepared by reversible addition–fragmentation transfer radical polymerization using a chain transfer agent bearing an n-dodecyl group and an azopyridine moiety, as described in detail previously.19 Their molar mass ranges from Mn 5800 g mol−1 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 (Table 1). Their chemical structure is presented in Scheme 1.
700 g mol−1 (Table 1). Their chemical structure is presented in Scheme 1.
| Sample name | Mn (g mol−1) a |
M
w/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
T cp/°C | T m/°C | ΔH/kJ | |||
|---|---|---|---|---|---|---|---|---|
| pH = 7 | pH = 3 | pH = 7 | pH = 3 | pH = 7 | pH = 3 | |||
| a M n and Mw/Mn were obtained by GPC with MALS detection (see ref. 14). b Data not available. | ||||||||
| C12-PN-AzPy 5 K | 5800 | 1.25 | 14.0 | 23.9 | 18.9 | —b | 2.7 | —b |
| C12-PN-AzPy 7 K | 7800 | 1.03 | 20.0 | 27.5 | 24.2 | 26.2 | 3.2 | 3.9 |
| C12-PN-AzPy 12 K | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.09 | 22.7 | 28.2 | 26.8 | 28.1 | 4.3 | 4.9 |
| C12-PN-AzPy 20 K | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.03 | 27.0 | 32.4 | 29.2 | 30.1 | 6.0 | 7.3 |
| C12-PN-AzPyC2H5+ 12 K | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.09 | 30.0 | 28.4 | 31.1 | 31.0 | 4.7 | 4.2 |
| C12-PN-Azo 12 K | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.23 | 22.5 | 19.5 | 26.6 | —b | 4.2 | —b |
Methods
Turbidity measurements. The cloud point temperatures of polymer aqueous solutions (1.0 mg mL−1) were determined using a UV-Vis spectrometer (Agilent 8452A) equipped with a temperature controller (Agilent 89090A). The heating rate was 0.5 °C min−1. Samples were equilibrated for 2 min at each temperature before measurement. The solution transmittance at 550 nm was measured as a function of the temperature from 15 °C to 60 °C. To determine the cloud point of polymer aqueous solutions subjected to UV light irradiation, the same protocol was performed with solutions subjected to constant irradiation at 365 nm with a LED-UV curing lamp (10% intensity, 30 mW cm−2). The cloud point (Tcp) was taken as the temperature of the onset of the decrease of the solution transmittance.Differential Scanning Calorimetry (DSC) measurements were performed on a VP-DSC microcalorimeter (MicroCal Inc.) with a cell volume of 0.520 mL. Three consecutive heating and cooling scans from 10–70 °C were performed with a heating and cooling rate of 1.0 °C min−1 using polymer solutions in water at a concentration of 1.0 mg mL−1.
1 H NMR spectra were recorded on a Bruker AMX-400 (400 MHz) spectrometer. Polymer solutions in D2O (2.0 mg mL−1) were brought to pH 10 by the dropwise addition of a 0.1 M NaOD solution. The samples were kept in a refrigerator for 24 h prior to measurement. For irradiation-dependent 1H NMR analysis, the polymer solutions were irradiated with a LED-UV curing lamp (365 nm, 10% intensity, 30 mW cm−2) for 30 s immediately prior to measuring their 1H NMR spectrum.
Evaluation of the photo/pH/temperature responsiveness of the polymer solutions
The pH- and photo-controlled thermoresponsive properties of polymer solutions were evaluated on a Mettler Toledo UV-Vis spectrophotometer (UV5 bio) equipped with a CuveT temperature controller. For experiments at constant temperature in the absence of UV light illumination, the absorbance of a solution of C12-PN-AzPy (1.0 mg mL−1) at 550 nm was recorded during a series of consecutives changes of the solution pH from pH 7 to pH 3 and from pH 7 to 10 using, respectively, 0.2 mM HCl or 0.2 mM NaOH aqueous solutions to adjust the solution pH. The same measurements were performed also for polymer solutions under constant UV irradiation (365 nm, 30 mW cm−2) at a constant temperature.Results and discussion
3.1 pH Dependence of the phase transition of aqueous trans-C12-PN-AzPy solutions
Table 1 presents key parameters of the heat-induced phase transition of C12-PN-AzPy aqueous solutions as a function of pH and the molar mass of the polymers in the absence of UV light irradiation. The Tcp values of aqueous C12-PN-AzPy solutions of pH 7 increase with increasing polymer molar mass (Fig. 1a), as a consequence of the increase of the hydrophilic polymer chain length while the length of the hydrophobic end group remains constant.26,39,40 The Tcp of C12-PN-Azo 12 K, a control sample that has an azobenzene end group instead of the AzPy group, is nearly identical to that of C12-PN-AzPy 12 K (22.5 °C vs. 22.7 °C), see Fig. S1.† The cloud points of C12-PN-AzPy solutions of pH 3, for which the azopyridine end group is protonated (pKa of azopyridine ∼4.52),41 are higher than those of the corresponding neutral solutions, as seen in Fig. 1b for solutions of C12-PN-AzPy 7 K. The amplitude of the increase in Tcp upon acidification, ΔTcp = Tcp (pH 3) − Tcp (pH 7) decreases with increasing polymer molar mass (see Fig. 1c, red bars). The Tcp of C12-PN-AzPyC2H5+ 12 K, which bears the cationic N-ethyl azopyridinium end group, is (28.4 °C, Fig. S1†) a value similar to that of C12-PN-AzPy 12 K in a pH 3 solution (28.2 °C). As a comparison, the Tcp values of aqueous solutions of unmodified PNIPAM of pH 3, 7, and 11 were determined as well (Fig. S2†). The Tcp values of the pH 3 and 11 solutions were ∼0.5 °C lower than those of the pH 7 solution. It is worth mentioning that the amide group of the NIPAM repeat units and the ester group that links the AzPy end group to the main chain withhold the experimental conditions, as reported previously according to previous reports.19,42High sensitivity microcalorimetry (HS-DSC) thermograms recorded for aqueous solutions of C12-PN-AzPy 7 K of pH 7.0 (black squares) and pH 3.0 (red circles) are presented in Fig. 1d, where Tm denotes the temperature corresponding to the maximum of the endotherm. The shift to a higher temperature of the transition maximum upon acidification (0.9 °C < ΔTm < 2.0 °C, with ΔTm = Tm (pH3) − Tm (p H7)) (Fig. 1c, blue bars) is smaller than ΔTcp. The enthalpy of the phase transition (ΔH, expressed in kJ mol−1 of NIPAM units) decreases with decreasing C12-PN-AzPy molar mass, especially in the case of neutral solutions (Table 1). This trend was observed also in earlier studies of aqueous solutions of neutral α, ω di-n-octadecyl-PNIPAM that forms core–shell flower micelles.43,44 Since the transition enthalpy corresponds to the release of polymer-bound water molecules into bulk water,45 a decrease in enthalpy indicates a lower degree of hydration of the PNIPAM corona, especially in the corona section close to the alkyl core as a result of the chain confinement. The ΔH values recorded for aqueous solutions of the longest polymer, C12-PN-AzPy 20 K in neutral and acidic solutions, 6.0 kJ mol−1 and 7.3 kJ mol−1, respectively, are similar to those of unmodified linear PNIPAM,44,46 Hence, C12-PN-AzPy 20 K exists in water in the form of free chains or loose aggregates, rather than core–shell micelles.
Comparing the turbidity curve recorded for the aqueous acidic C12-PN-AzPy 7 K (Fig. 1b, red curve) to the thermogram of this solution (Fig. 1d, red curve), we observe that the Tcp value of this solution, 27.5 °C, is higher than Tm (26.2 °C). The azopyridinium ions stabilize the dehydrated/collapsed chains against macroscopic aggregation,36 but their stabilizing effect is confined within a narrow temperature window (∼1–2 °C above T). Upon further heating, solutions become turbid indicating the formation of large mesoglobules. We surmise, that as the temperature increases, the hydrophobic AzPy+ groups relocate within the collapsed chains decreasing the electrostatic repulsion between collapsed globules. In the case of the neutral C12-PN-AzPy (pH 7) solutions, Tcp nearly coincides with the endotherm onset temperature, which confirms that under neutral conditions the clouding of the solution and the dehydration/collapse of the PNIPAM chains occur at about the same temperature (Fig. 1d). The Tcp values of polymer solutions of pH 10 are slightly lower than the corresponding values for solutions of pH 7, which can be attributed to the presence of OH− ions.47,48 (see turbidity plots in Fig. S3†).
3.2. Combined effects of pH and UV light irradiation on the phase transition of aqueous C12-PN-AzPy solutions
UV light photoirradiation of trans AzPy yields the less stable cis AzPy that thermally relaxes back to the trans form. As described above, the cis–trans thermal relaxation of AzPyH+ in C12-PN-AzPyH+ micelles (pH 3) is on the order of a few ms as determined by transient absorption spectroscopy (Scheme 1b, box pH = 3 inset). In neutral solutions, the cis–trans dark relaxation is on the order of a few seconds, since the flower-like morphology of C12-PN-AzPy enables the formation of H-bonds between the AzPy nitrogen and the amide hydrogen of the surrounding PNIPAM repeat units. In a basic solution, the dark cis to trans relaxation of AzPy takes several hours since the hydrogen bonds between the AzPy nitrogen and the PNIPAM amide groups are broken by the excess hydroxyl ions competing with the AzPy nitrogen as hydrogen bond acceptors.19 Cis-C12-PN-AzPy is sufficiently long-lived in alkaline aqueous solutions for the characterization by NMR spectroscopy, turbidimetry, and steady state absorption spectroscopy (Fig. S4†).The 1H NMR spectra of C12-PN-AzPy in D2O (pH 10) before irradiation and after 30 s irradiation at 365 nm are presented in Fig. 2a and b, respectively. Then trans-to-cis isomerization induces significant changes of the aromatic proton resonances. The protons meta to the pyridine nitrogen and the protons on the phenyl ring experience large upfield shifts from 7.79, 7.54, and 6.95 ppm (labeled b, c, and d) to ∼6.86 ppm. The protons ortho to the pyridine nitrogen (labeled a in Fig. 2) undergo a small upfield shift from 8.63 to 8.45 ppm. In addition, the total area of the signals due to the aromatic protons, normalized to the area of the signal at 3.85 ppm due to the NIPAM methine protons increases by 48.3% (from 0.060 for trans-C12-PN-AzPy to 0.089 for the cis isomer). The upfield shift of the aromatic proton resonances reflects the decrease of the π-electron delocalization in the non planar cis AzPy, compared to trans AzPy.37 The apparent increase of the aromatic resonance intensity is an indication of the enhanced mobility of the aromatic rings of cis-AzPy compared to trans-AzPy, possibly as a result of a higher level of hydration of the cis AzPy vs. the trans AzPy. Similar 1H NMR results were observed in aqueous solutions of C12-PN-AzPy 12 K (Fig. S5†).
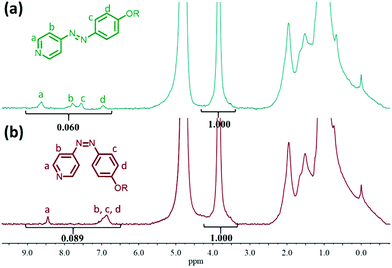 | ||
| Fig. 2 1H-NMR spectrum of C12-PN-AzPy 7 K (a) before and (b) after UV irradiation at pH = 10 (1.0 mg mL−1 in D2O, 18.5 °C). | ||
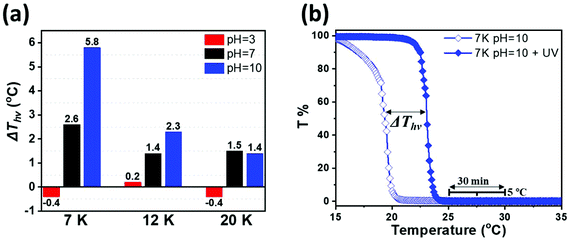
The cloud points of pH 10 aqueous solutions of cis-C12-PN-AzPy were obtained by measuring the changes in the solution transmittance at 550 nm as a function of temperature under constant irradiation at 365 nm. The resulting turbidity plots of solutions of cis-C12-PN-AzPy 7 K (full symbols) and trans-C12-PN-AzPy 7 K (open symbols) are presented in Fig. 3b. The cloud point of the cis form is significantly higher than that of the trans form, with ΔThν = Tcp (cis) − Tcp (trans) ∼ 5.8 °C. The ΔThν value decreases with increasing PNIPAM molar mass, due to the increase of the NIPAM units/AzPy ratio (see Table 2) presented in Fig. S6.† The increase in the hydration, observed by 1H NMR spectroscopy, and larger dipole moment of cis-AzPy, compared to trans-AzPy, are contributing factors causing the increase of Tcp (cis).49
| Sample name | pH | T cp before UV/°C | T cp after UV/°C | ΔThv/°C |
|---|---|---|---|---|
| C12-PN-AzPy 7 K | 3 | 27.5 | 27.1 | −0.4 |
| 7 | 20.0 | 22.6 | 2.6 | |
| 10 | 16.0 | 21.8 | 5.8 | |
| C12-PN-AzPy 12 K | 3 | 28.2 | 28.4 | 0.2 |
| 7 | 22.7 | 24.1 | 1.4 | |
| 10 | 22.0 | 24.3 | 2.3 | |
| C12-PN-AzPy 20 K | 3 | 32.4 | 32.0 | −0.4 |
| 7 | 27.0 | 28.5 | 1.5 | |
| 10 | 26.8 | 28.2 | 1.4 | |
| C12-PN-AzPyC2H5+ 12 K | 7 | 30.0 | 30.4 | 0.4 |
| C12-PN-Azo 12 K | 7 | 22.5 | 24.5 | 2.0 |
We measured also turbidity plots of C12-PN-AzPy aqueous solutions of pH 3 and 7 before irradiation (trans form) and under continuous UV irradiation. For solutions of pH 3, the Tcp values recorded during irradiation were identical to the corresponding Tcp (trans), as anticipated since the short-lived cis isomer cannot be detected by turbidity measurements. The Tcp of C12-PN-AzPyC2H5+ during irradiation, also, is identical to that recorded for trans-C12-PN-AzPyC2H5+ (see Table 2). A small increase of Tcp upon irradiation was observed for C12-PN-AzPy in neutral aqueous solutions, which indicates that the irradiated solutions contain a small, but detectable, fraction of cis-C12-PN-AzPy throughout the measurement.
Scheme 2 presents an overview of the properties and appearance of aqueous solutions (pH 3, pH 7, and pH 10) of AzPy capped PNIPAMs upon UV irradiation and/or upon heating and the different time scales of the phenomena involved.
In neutral or basic aqueous solutions of C12-PN-AzPy (blue and green frames) the polymers adopt a core–shell micelle morphology. Upon heating in the dark, the PNIPAM chains of the shell dehydrate and collapse resulting in the formation of smaller hydrophobic micelles that aggregate into larger particles, observed visually by the appearance of turbidity (top row vials in each frame). Upon short UV irradiation, the turbid solutions become transparent, since light converts trans-C12-PN-AzPy into cis-C12-PN-AzPy, which has a higher Tcp. In the dark, solutions of pH 7 become turbid again within a few seconds. The recovery is very slow in the case of solutions of pH 10.
In solutions of pH 3 (yellow frame), C12-PN-AzPyH+ chains assemble in star cationic micelles. The heat induced dehydration of PNIPAM results in the formation of small aggregates of collapsed micelles stabilized electrostatically, barely detectable visually, that increase in size upon further heating.36 Constant UV irradiation has no effect on the visual appearance of pH 3 C12-PN-AzPyH+ solutions at a given temperature, since the trans and cis isomers undergo fast exchange.
Based on the above, we can design complex “isothermal” phase transition systems by simply changing the solution pH or applying UV irradiation. For example, we can control the solution turbidity at a constant temperature by consecutives changes of the solution pH from pH 7 to pH 3 or to pH 10 using 0.2 mM HCl or 0.2 mM NaOH aqueous solutions, see Fig. S7.† An increase of the pH from 7 to 10 will instantly stop the UV on/off reversible isothermal transition, and the reversibility is recovered upon decreasing the solution pH from 10 to 7. Various permutations of the trigger sequences can be envisaged in specific situations.
Conclusion
The ionization of AzPy end groups, the trans–cis photoisomerization of AzPy, and the kinetics of the cis–trans relaxation over time ranging from hours to a few ms influence the thermoresponsive behavior of the polymers, each in a specific way. Ionization of the azopyridine end group stabilizes the dehydrated chains against macroscopic aggregation within a specific temperature range (∼1–2 °C above Tm). Of particular importance from the practical view point, the UV induced turbidity of the polymeric solutions changes as a function of pH, as a consequence of the pH-dependent kinetics of the cis to trans dark relaxation of AzPy. This study contributes to the fundamental understanding of the effects of end group ionization and photosensitivity on the macroscopic phase transition of modified PNIPAMs. It leads the way to simple strategies for the use of AzPy functional amphiphilic polymers in aqueous fluids, hydrogels, or films as sensors or actuators controllable by the application of light and changes of temperature and pH in permutable sequences.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
HR. thanks the China Postdoctoral Science Foundation Grant (No. 2019M653859XB). FMW acknowledges the Natural Sciences and Engineering Research Council of Canada for partial support of this work.References
- H. V. Zollinger, Color chemistry: syntheses, properties, and applications of organic dyes and pigments, VCH, Weinheim, 1991 Search PubMed.
- G. S. Kumar and D. C. Neckers, Chem. Rev., 1989, 89, 1915–1925 CrossRef CAS.
- K. Dušek and M. Ilavský, Responsive Gels: Volume Transitions II, Springer, 1993, vol. 110, pp. 49–65 Search PubMed.
- A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139–4176 CrossRef CAS PubMed.
- H. M. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- J. Griffiths, Chem. Soc. Rev., 1972, 1, 481–493 RSC.
- H. Ren, D. Chen, Y. Shi, H. Yu and Z. Fu, Polym. Chem., 2015, 6, 270–277 RSC.
- S. Sun, S. Liang, W. Xu, G. Xu and S. Wu, Polym. Chem., 2019, 10, 4389–4401 RSC.
- K. Bujak, H. Orlikowska, J. G. Małecki, E. Schab-Balcerzak, S. Bartkiewicz, J. Bogucki, A. Sobolewska and J. Konieczkowska, Dyes Pigm., 2019, 160, 654–662 CrossRef CAS.
- R. Dong, B. Zhu, Y. Zhou, D. Yan and X. Zhu, Polym. Chem., 2013, 4, 912–915 RSC.
- F. D. Jochum and P. Theato, Chem. Commun., 2010, 46, 6717–6719 RSC.
- A. H. Gelebart, D. J. Mulder, M. Varga, A. Konya, G. Vantomme, E. W. Meijer, R. L. B. Selinger and D. J. Broer, Nature, 2017, 546, 632–636 CrossRef CAS PubMed.
- D. Schulte-Frohlinde, Justus Liebigs Ann. Chem., 1958, 612, 138–152 CrossRef.
- B. C. Buddingh and J. C. M. van Hest, Acc. Chem. Res., 2017, 50, 769–777 CrossRef CAS PubMed.
- T. Suzuki, T. Moriya, R. Endo and N. Iwasaki, Polym. Chem., 2017, 8, 761–768 RSC.
- Y. Ni, X. Li, J. Hu, S. Huang and H. Yu, Chem. Mater., 2019, 31, 3388–3394 CrossRef CAS.
- J. Wei, Z. Yan, L. Lin, J. Gu, Z. Feng and Y. Yu, React. Funct. Polym., 2013, 73, 1009–1014 CrossRef CAS.
- K. Aoki, M. Nakagawa and K. Ichimura, J. Am. Chem. Soc., 2000, 122, 10997–11004 CrossRef CAS.
- H. Ren, X. Qiu, Y. Shi, P. Yang and F. M. Winnik, Macromolecules, 2019, 52, 2939–2948 CrossRef CAS.
- S. Fujishige, K. Kubota and I. Ando, J. Phys. Chem., 1989, 93, 3311–3313 CrossRef CAS.
- L. D. Taylor and L. D. Cerankowski, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 2551–2570 CrossRef CAS.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342–15367 CrossRef CAS PubMed.
- J. Y. Quek, Y. Zhu, P. J. Roth, T. P. Davis and A. B. Lowe, Macromolecules, 2013, 46, 7290–7302 CrossRef CAS.
- J. He, L. Tremblay, S. Lacelle and Y. Zhao, Polym. Chem., 2014, 5, 5403–5411 RSC.
- K. Otake, H. Inomata, M. Konno and S. Saito, Macromolecules, 1990, 23, 283–289 CrossRef CAS.
- Y. Xia, N. A. D. Burke and H. D. H. Stöver, Macromolecules, 2006, 39, 2275–2283 CrossRef CAS.
- Y. Zhao, Macromolecules, 2012, 45, 3647–3657 CrossRef CAS.
- J. Qian and F. Wu, J. Mater. Chem. B, 2013, 1, 3464–3469 RSC.
- X. Huang, X. Jiang, Q. Yang, Y. Chu, G. Zhang, B. Yang and R. Zhuo, J. Mater. Chem. B, 2013, 1, 1860–1868 RSC.
- M. J. Summers, D. J. Phillips and M. I. Gibson, Chem. Commun., 2013, 49, 4223–4225 RSC.
- D. J. Phillips, I. Prokes, G. Davies and M. I. Gibson, ACS Macro Lett., 2014, 3, 1225–1229 CrossRef CAS.
- X. Jiang, C. Feng, G. Lu and X. Huang, ACS Macro Lett., 2014, 3, 1121–1125 CrossRef CAS.
- J. R. Lovett, N. J. Warren, L. P. D. Ratcliffe, M. K. Kocik and S. P. Armes, Angew. Chem., Int. Ed., 2015, 54, 1279–1283 CrossRef CAS PubMed.
- G. Haberhauer, C. Kallweit, C. W. Lper and D. Bl Ser, Angew. Chem., Int. Ed., 2013, 52, 7879–7882 CrossRef CAS PubMed.
- H. Ren, D. Chen, Y. Shi, H. Yu, Z. Fu and W. Yang, Macromolecules, 2018, 51, 3290–3298 CrossRef CAS.
- P. A. FitzGerald, S. Gupta, K. Wood, S. Perrier and G. G. Warr, Langmuir, 2014, 30, 7986–7992 CrossRef CAS PubMed.
- A. O. Moughton and R. K. O'Reilly, Chem. Commun., 2010, 46, 1091–1093 RSC.
- A. O. Moughton, J. P. Patterson and R. K. O'Reilly, Chem. Commun., 2011, 47, 355–357 RSC.
- X. Qiu, T. Koga, F. Tanaka and F. M. Winnik, Sci. China: Chem., 2013, 56, 56–64 CrossRef CAS.
- J. Du, H. Willcock, J. P. Patterson, I. Portman and R. K. O'Reilly, Small, 2011, 7, 2070–2080 CrossRef CAS PubMed.
- E. Buncel and S. Keum, Tetrahedron, 1983, 39, 1091–1101 CrossRef CAS.
- T. Hoare and R. Pelton, Langmuir, 2004, 20, 2123–2133 CrossRef CAS.
- T. Koga, F. Tanaka, R. Motokawa, S. Koizumi and F. M. Winnik, Macromolecules, 2008, 41, 9413–9422 CrossRef CAS.
- P. Kujawa, F. Segui, S. Shaban, C. Diab, Y. Okada, F. Tanaka and F. M. Winnik, Macromolecules, 2006, 39, 341–348 CrossRef CAS.
- E. I. Tiktopulo, V. E. Bychkova, J. Ricka and O. B. Ptitsyn, Macromolecules, 1994, 27, 2879–2882 CrossRef CAS.
- X. Qiu, F. Tanaka and F. M. Winnik, Macromolecules, 2007, 40, 7069–7071 CrossRef CAS.
- M. E. Tuckerman, D. Marx and M. Parrinello, Nature, 2002, 417, 925–929 CrossRef CAS PubMed.
- K. Abu-Dari, K. N. Raymond and D. P. Freyberg, J. Am. Chem. Soc., 1979, 101, 3688–3689 CrossRef CAS.
- C. Brown, S. K. Rastogi, S. L. Barrett, H. E. Anderson, E. Twichell, S. Gralinski, A. McDonald and W. J. Brittain, J. Photochem. Photobiol., A, 2017, 336, 140–145 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The detail of cloud point test influenced by pH values and UV irradiation, as well as the 1H-NMR spectrum of C12-PN-AzPy 12 K after UV irradiation. See DOI: 10.1039/c9py01086f |
| This journal is © The Royal Society of Chemistry 2019 |