 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of functional diols derived from pentaerythritol as chain extenders in the synthesis of novel thermoplastic polyester-urethane elastomers†
Ruairí P.
Brannigan
 ab,
Anthony
Walder
c and
Andrew P.
Dove
ab,
Anthony
Walder
c and
Andrew P.
Dove
 *d
*d
aDepartment of Chemistry, University of Warwick, Gibbet Hill Rd, Coventry, CV4 7AL, UK
bDepartment of Chemistry, RCSI, 123 St. Stephens Green, Dublin 2, D02 YN77, Ireland
cThe Lubrizol Corporation, 207 Lowell Street, Wilmington, Massachusetts 01887, USA
dSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. E-mail: a.dove@bham.ac.uk
First published on 9th September 2019
Abstract
The success of thermoplastic elastomers is owed to the wide range of thermal, mechanical and degradation properties that enable them to be tuned to myriad applications. In large, this is achieved through the manipulation of the chemical structure of their component parts e.g. polyol, diisocyanate and diol chain extender. The permutation of diol extenders is of great interest industrially owing to their commercial availability and ease of synthesis and purification, however, the incorporation of extenders with post-polymerisation modifiable sites, specifically moieties prone to ‘click’ coupling chemistries has been relatively overlooked until recent times. Herein, the application of ‘acetal-diols’, (2-phenyl-1,3-dioxane-5,5-diyl)dimethanol (CPh) and (2-(norbornene)-1,3-dioxane-5,5-diyl)dimethanol (CNb), as extenders in the synthesis of novel thermoplastic polyurethanes (TPUs) is described. The assessment of their structure–property relationship reveals that the material properties are highly tailorable based on their urethane content. Furthermore, norbornene ‘click’ chemistries could be utilised post-polymerisation as a method of manipulating the materials’ hydrophilicity and degradability.
Introduction
Characterised by their enhanced ability to recover after incurring high strains and deformation, thermoplastic elastomeric materials have been of great interest in both biomedical and wider industrial applications.1–3 Of this class of materials, thermoplastic polyurethanes (TPUs) are arguably the most widely investigated and utilised. This category of segmented elastomeric polymers is generally synthesised via the step-growth polymerisation of telechelic polymeric diols (polyols) with diisocyanates and small diol ‘chain-extenders’, however, more recently analogues of these systems have been synthesised through the ring-opening polymerisation of cyclic carbonates.4–7 The success of elastomeric TPUs is owed to their ability to access a vast range of thermal and mechanical properties through the mediation of their component parts. Most notable of these is the incorporation of hydroxyl terminated telechelic polyesters (i.e. poly(ε-caprolactone) (PCL), poly(lactide-co-glycolide) (PLGA), poly(trimethylene carbonate) (PTMC) etc.) as the polyol, yielding hydrolytically degradable thermoplastic polyester urethanes (TPEUs).8–10 Equally, the permutation of diol extenders is of great interest industrially owing to their commercial availability and ease of synthesis and purification. The design of novel small diol extenders in order to control the physical properties of the resultant TPEUs has been extensively investigated since their inception, however, the application of extenders with post-polymerisation modifiable sites has been relatively overlooked until recent times.11,12So-called ‘click’ reactions have been extensively utilised in polymer chemistry as an efficient method for the conjugation of target molecules onto polymeric constructs.13–15 Although initially outlined under criteria set out by Kolb, Finn and Sharpless, the definitive qualities of ‘click’ reactions have been modified since their application in polymer chemistry.16 In relation to polymer chemistry, ‘click’ reactions must be equimolar, allow for facile large scale purification, proceed in a rapid manner, be high yielding and utilise stable products.16 One moiety which has been extensively studied, owing to its ability to undergo a selection of ‘click’ reactions, is norbornene. Norbornene moieties can efficiently undergo 1,3-dipolar cycloaddition with azides, inverse electron-demand Diels–Alder (DAinv) reactions with tetrazines and photoinduced radical thiol addition reactions in addition to several non-‘click’ conjugation methods.17–22
The incorporation of cyclic acetal moieties into polymeric systems represents a very important advancement in the development of biocompatible, hydrolytically degradable and pH responsive materials.23–25 Cyclic acetals are therefore excellent candidates in the synthesis of pH responsive drug delivery vectors. Recently, we have shown that the incorporation of norbornene-acetal moieties in an amphiphilic polycarbonate-g-poly(ethylene glycol) system yielded materials which could be easily modified through ‘click’-like chemistry and subsequently used as a pH responsive chemotherapeutic delivery system.26 Herein, we describe the application of previously synthesised ‘acetal-diols’, (2-phenyl-1,3-dioxane-5,5-diyl)dimethanol (CPh) and (2-(norbornene)-1,3-dioxane-5,5-diyl)dimethanol (CNb), as extenders in the synthesis of novel TPEUs and assess their structure–property relationship. Furthermore, the post-polymerisation modification of the norbornene-containing TPEUs, as a method of manipulating their physical properties, is investigated.
Results and discussion
As has been previously reported, the acid-catalysed acetal formation between aldehydes and diols offers a facile and efficient route to synthesise monomers and polymers that contain pH sensitive cyclic acetal moieties.26,27 Acetal-containing diols were generated through the reaction of pentaerythritol with benzaldehyde and norbornene-2-carboxaldehyde under acidic conditions respectively. The utilisation of deionised water as the reaction solvent allowed for the precipitation of the mono-functionalised product from the reaction exclusively (Fig. S1†), owing to the increased hydrophobicity of the molecule through the introduction of the benzyl and norbornene moieties. The conversion of pentaerythritol to the benzylidene acetal functional diol (CPh) was confirmed by 1H and 13C NMR spectroscopy. From the 1H NMR spectrum, the evolution of a multiplet at δ = 7.45–7.31 ppm and a singlet at δ = 5.40 ppm, attributed to the benzyl functionality and the methine adjacent to the acetal respectively, verify the successful modification of the pentaerythritol. This is further confirmed by the presence of two triplets at δ = 4.57 and 4.66 ppm attributed to the protons of the diol hydroxyl groups (Scheme 1). Similarly, the synthesis of (2-(norbornene)-1,3-dioxane-5,5-diyl)dimethanol (CNb) was also verified by 1H and 13C NMR spectroscopy through the presence of a series of signals between δ = 3.00 ppm and 0.60 ppm, attributed to the saturated norbornene hydrocarbons (Fig. S1†).Furthermore, the presence of two multiplets at δ = 6.17 ppm and 5.94 ppm confirms the retention of the norbornene alkene functionality (Fig. S1†). As has been previously reported, the most utilised method in the synthesis of TPUs is the ‘2-shot’ method, owing to the formation of highly segmented thermoplastic polyester urethanes (TPEUs).28,29 This method involves the formation of a polyester-based ‘pre-polymer’ by the reaction of the polyol with an excess of diisocyanates. This yields isocyanate end-capped polyesters which are subsequently polymerised by step-growth methods by the addition of a small diol extender. Hydroxy-terminated telechelic poly(ε-caprolactone) with a weight-average molecular weight (Mw) = 2000 g mol−1 (PCL2k), as determined by size exclusion chromatography (SEC), was selected as the polyol and 1-isocyanato-4-[(4-isocyanatocyclohexyl)methyl] cyclohexane (H12MDI) was chosen as the diisocyanate. The weight percentage of the overall TPEU that is ‘hard segment’ (%HS) was determined based on the overall urethane content, and TPEUs with varying target %HS of 30, 45 and 60% were synthesised. The molar ratio of diisocyanate to polyol was calculated using eqn (S1).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 By altering the composition of the TPEUs by varying the %HS and extender used, it is possible to tune the morphology of these segmented materials and in turn the mechanical properties.30 In addition, owing to its relatively low toxicity in comparison to conventional tin-based catalysts, 1,8-diazabicycloundec-7-ene (DBU) was employed as an organocatalyst for the step-growth polymerisation through the activation of the polyol/extender hydroxyl groups, aiding in the nucleophilic attack of the isocyanate bonds. The polymerisation was monitored by Fourier-transform infra-red (FT-IR) spectroscopy through the reduction of absorption bands attributed to the cumulated double bonds of the diisocyanate at ∼2275 cm−1 and ∼2250 cm−1. Furthermore, it is inferred the complete consumption of the diisocyanate is indicative of full incorporation of the diol chain extender. SEC analysis revealed that the CPh and CNb-based TPEUs exhibited unimodal weight distributions with dispersities (ĐM) close to 2 (ĐM ≈ 2.1–2.4), characteristic of step growth polymerisations (Table 1 and Fig. 1).31 Interestingly, it was noted that the dispersities of the TPEUs increased, while the Mw decreased, with increasing %HS. It is hypothesised that this is a consequence of the increased viscosity of the polymerisation reaction with higher molar ratios of diisocyanate/extender to polyol, reducing the availability of reactive chain ends.
28 By altering the composition of the TPEUs by varying the %HS and extender used, it is possible to tune the morphology of these segmented materials and in turn the mechanical properties.30 In addition, owing to its relatively low toxicity in comparison to conventional tin-based catalysts, 1,8-diazabicycloundec-7-ene (DBU) was employed as an organocatalyst for the step-growth polymerisation through the activation of the polyol/extender hydroxyl groups, aiding in the nucleophilic attack of the isocyanate bonds. The polymerisation was monitored by Fourier-transform infra-red (FT-IR) spectroscopy through the reduction of absorption bands attributed to the cumulated double bonds of the diisocyanate at ∼2275 cm−1 and ∼2250 cm−1. Furthermore, it is inferred the complete consumption of the diisocyanate is indicative of full incorporation of the diol chain extender. SEC analysis revealed that the CPh and CNb-based TPEUs exhibited unimodal weight distributions with dispersities (ĐM) close to 2 (ĐM ≈ 2.1–2.4), characteristic of step growth polymerisations (Table 1 and Fig. 1).31 Interestingly, it was noted that the dispersities of the TPEUs increased, while the Mw decreased, with increasing %HS. It is hypothesised that this is a consequence of the increased viscosity of the polymerisation reaction with higher molar ratios of diisocyanate/extender to polyol, reducing the availability of reactive chain ends.
 | ||
| Fig. 1 Size exclusion chromatograms of (i) CPh and (ii) CNb-based TPEUs in DMF against PMMA standards (Table 1). | ||
| Polymer | wt% ‘hard block’ (%HS) | H12MDI/polyol ratioa (R) |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (kg mol−1) b (kg mol−1) |
Đ
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|
| a Determined by eqn (S1).† b Determined by GPC analysis in DMF against poly(methyl metacrylate) (PMMA) standards. | ||||
| 30CPh | 30 | 2.99 | 97.8 | 2.18 |
| 45CPh | 45 | 4.80 | 97.0 | 2.23 |
| 60CPh | 60 | 7.97 | 88.5 | 2.30 |
| 30CNb | 30 | 2.93 | 99.0 | 2.19 |
| 45CNb | 45 | 4.68 | 108.9 | 2.17 |
| 60CNb | 60 | 7.75 | 90.1 | 2.38 |
The final composition of the CPh and CNb-based TPEUs, as calculated by eqn (S1),† were verified by 1H NMR and FT-IR spectroscopy. Expectedly, it was found by 1H NMR spectroscopy, for both CPh and CNb-based materials, that the signals attributed to the PCL ‘soft’ block (δ = 3.98, 2.27, 1.52 and 1.29 ppm) decreased with increasing %HS. In conjunction with this, the characteristic signals attributed to the functionalised pentaerythritol-derived extenders (CPh; δ = 7.36 and 5.45 ppm, δ = CNb; 6.30–5.80 ppm) were found to increase proportionally with increasing %HS, indicative of good extender incorporation into the final materials (Fig. S2†). Furthermore, the FT-IR spectra of both the CPh and CNb-based TPEUs displayed absorption bands attributed to the urethane carbonyl stretching (amide I) and N–H bending (amide II) frequencies at ∼1700 and ∼1500 cm−1 respectively. Analogous to the trend observed in the 1H NMR spectra, it was found that these absorptions increased proportionally to the %HS. Importantly, it was noted that the absence of an absorbance band between 2250 and 2275 cm−1, attributed to the isocyanate cumulative double bonds, is indicative of complete consumption of the H12MDI (Fig. S3†). Owing to differences in scattering patterns of contrasting polymers in segmented block copolymer systems, wide angle X-ray diffraction (WAXD) analysis can be used to determine phase separation within a material.32–34
As such, in order to assess the morphology of the materials and verify the synthesis segmented TPEUs, annealed materials were subjected to WAXD analysis. For both CPh and CNb extenders, it was found that the X-ray diffraction patterns displayed a series of sharp crystalline peaks with a Bragg's angle (2θ) between 5–30°, however, the resultant TPEUs were found to display two broad undefined peaks at 2θ ≈ 20° and 2θ ≈ 44°. This is indicative of phase separated amorphous domains attributed to the ‘hard’ polyurethane segment and the ‘soft’ PCL segment respectively.35,36 (Fig. S4†).
In order to assess the tensile properties of the CPh and CNb-based TPEUs in relation to their composition, annealed materials with varying %HS were subjected to axial loading at a constant crosshead speed (5 mm min−1) until failure. From an average of 10 comparable samples, the Young's modulus (E), elongation at break (εbreak) and ultimate tensile strength (UTS) of the materials were determined (Table 2). The tensile analysis of the TPEUs expectedly displayed that both UTS and E increased proportionately with %HS, however, εbreak was found to be inversely proportional. As is evident from previous synonymous studies, this is a consequence of increased hydrogen bonding through the urethane moieties of the ‘hard’ segments, yielding brittle materials. It was noted that both the CPh and CNb-based TPEUs with a 45% ‘hard-block’ content (45CPh and 45CNb, Table 2), displayed non-linear viscoelastic behaviour with relatively good elongation before mechanical failure (420–460% strain) and underwent recoverable deformation at 30–40% strain. Furthermore, at high %HS (60%) it was found that TPEUs underwent plastic deformation and mechanical failure at low strains (<45%) but exhibited a high UTS (>29 MPa).
| Polymer | E (MPa) |
ε
break![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
UTSa (MPa) |
|---|---|---|---|
| a Determined by tensiometric analysis (average of 10 samples, Fig. 2). | |||
| 30CPh | 8.4 ± 1.4 | 757 ± 10 | 5.9 ± 0.16 |
| 45CPh | 39.6 ± 2.8 | 424 ± 9 | 24.4 ± 0.44 |
| 60CPh | 109.8 ± 5.6 | 41 ± 3 | 29.6 ± 1.01 |
| 30CNb | 4.6 ± 0.9 | 1130 ± 19 | 2.2 ± 0.26 |
| 45CNb | 40.2 ± 2.0 | 462 ± 12 | 25.3 ± 0.86 |
| 60CNb | 110.3 ± 6.8 | 47 ± 2 | 30.5 ± 1.30 |
Conversely to this, at lower %HS (30%), TPEUs were found to have high elongation before mechanical failure (760–1130% strain) but exhibited extended recovery times after undergoing elastic deformation with low E (5–8 MPa) and UTS (2–6 MPa) (Fig. 2). CPh and CNb-based TPEUs were subjected to accelerated degradation conditions in order to assess their hydrolytic degradability. Each of the TPEUs were compression moulded into degradation ‘disks’ and placed into individual vials before being submerged in basic aqueous media (5 M aq. NaOH). The degradation samples were stored at 37 °C with constant agitation at 60 rpm and the weights of the dried disks were measured periodically with an analytical balance until they were irretrievable from the degradation media. This method was chosen to exhibit the hydrolytic degradation of the TPEUs as it allowed for the most rapid analysis and comparison of the degradation properties of the materials. The accelerated degradation was performed in triplicate and all results were reported as an average. For both CPh and CNb-based TPEUs, it was noted that differences in the overall percentage swelling and rate of degradation observed were found to be negligible, irrespective of the side group functionality (maximum swelling 60CPh = 14 ± 0.6%, 60CNb = 11 ± 0.8%, Fig. 3). Furthermore, it was found that the percentage swelling increased slightly with increasing %HS. It is hypothesised that this is owing to the decrease in the hydrophobic PCL domains, generating a more hydrophilic material. In order to verify this hypothesis, static contact angle measurements with water were conducted on thin films of the TPEUs. The contact angle of a material is defined as the angle of a liquid/vapour interface when in contact with a solid surface. This angle can therefore be used to infer the relative hydrophilicity/hydrophobicity of a surface owing to differences in the interfacial tension caused by the surface composition. If the contact angle is greater than 90° the surface is said to be non-wetting (hydrophobic), whereas a contact angle of less than 90° denotes a surface which is said to be wettable (hydrophilic) with an angle of 0° which indicates complete wetting.12 Concurrent with the accelerated degradation study, it was found that the difference in contact angle measurements were relatively low when side-group functionality was changed from phenyl to norbornene acetal (Fig. S5†). Moreover, the contact angle measurements verified the increase in hydrophilicity with increasing %HS hypothesised in the accelerated degradation study (Scheme 2).
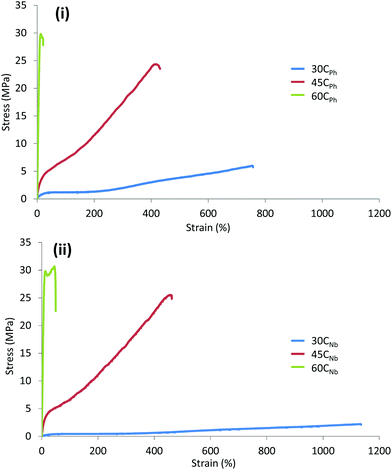 | ||
| Fig. 2 Exemplar stress–strain curves of (i) CPh-based TPEUs and (ii) CNb-based TPEUs. Experiments were conducted at ambient temperature (∼25 °C) at an elongation rate of 5 mm min−1 until failure. | ||
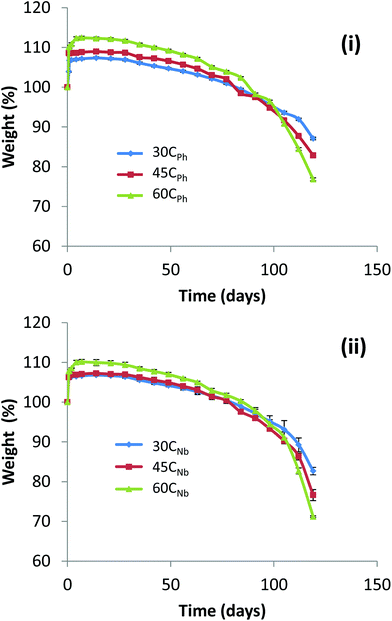 | ||
| Fig. 3 Hydrolytic degradation of (i) CPh and (ii) CNb-based TPEUs under accelerated degradation conditions. Percentage mass loss of an average of three samples. | ||
 | ||
| Scheme 2 The post-polymerisation functionalisation of the norbornene acetal moieties of CNb-based TPEUs. | ||
As has been demonstrated in the literature, the post-polymerisation modification of norbornene-containing polymers offers a unique method of creating novel materials with various properties and applications.22 As such, in order to assess the effect the modification of CNb-based TPEUs with hydrophilic small molecules has on the overall hydrophilicity and degradation profile of the TPEUs, CNb-based TPEUs were modified via a 1,3-dipolar cycloaddition with O-(2-azidoethyl)-O′-methyl-triethylene glycol (TEG-azide) and the photoinduced radical thiol addition with 1-thioglycerol. Furthermore, CNb-based TPEUs were modified with the fluorophore, 7-mercapto-4-methylcoumarin (MMC), in order to demonstrate the efficiency of the modification process via ultraviolet-visible (UV-Vis) spectrophotometry. The 1H NMR spectra of the modified CNb-based TPEUs reveal the complete consumption of the norbornene alkene functionality at δ = 5.94 and 6.17 ppm and the appearance of characteristic signals attributed to each modification (TEG-azide; δ = 3.50 ppm, 1-thioglycerol; δ = 4.50–5.00 ppm, 7-mercapto-4-methylcoumarin; 7.75–6.75 and 7.20 ppm, Fig. S6†), indicative of successful post-polymerisation modification. The modified TPEUs were analysed by GPC and were found to increase in molecular weight with no significant effect on the dispersities, indicative of no adverse side reactions i.e. chain–chain coupling, side-group cleavage etc. (Fig. S7†). Additionally, the successful modification of 45CNb with the fluorophore MMC (45CNb-MMC) was also verified by UV-vis spectrophotometry. It was found that the unmodified 45CNb exhibited no absorbance peak, however, after modification, a peak at λ = 388 nm was observed characteristic of the absorbance of 7-mercapto-4-methylcoumarin. Furthermore, it was noted that by reducing the equivalents of MMC used during the photoinduced thiol addition (1.1 eq. to 0.51 eq.) it was possible to control the incorporation of fluorophore into the molecule. This was denoted by a reduction in the intensity of absorbance observed, in accordance with the Beer–Lambert law (Fig. S8†).
In order to assess the effect that the modification of CNb-based TPEUs with hydrophilic small molecules has on the swelling/degradation profiles and surface hydrophilicity, thioglycerol (45CNb-gly) and O-(2azidoethyl)-O′-methyl-triethylene glycol (45CNb-TEG) modified TPEUs were subjected to accelerated degradation studies and static contact angle measurements as previously described. As anticipated, the incorporation of hydrophilic molecules into the ‘hard’ polyurethane segments led to an increase in the surface hydrophilicity of the materials observable by a severe reduction in the static contact angle measurements from 78.8° to 59.1° (Fig. S9†). Furthermore, the accelerated degradation study revealed that the percentage swelling of the modified TPEUs increased from 7% to 13% after modification, indicative of an increased ingression of the aqueous degradation media into the TPEU network owed to increased hydrophilicity (Fig. S10†). In conjunction with the increase in percentage swelling, it was noted that a slightly higher rate of weight loss was also observed. The increased rate of weight loss is indicative of a higher hydrolytic degradation rate owed to the increased concentration of degradation media at hydrolysable bonds.
Finally, in order to assess the effect the post-polymerisation modifications had on the mechanical properties of the materials, modified 45CNb-based TPEUs were analysed by tensiometric analysis. Furthermore, in order to generate consistent results, all of the modified TPEUs were processed, annealed and analysed under the same conditions. From tensiometric analysis, it was found that there was no significant change in the tensile properties of the 45CNb-gly TPEU in comparison with the unmodified TPEU, whilst the 45CNb-TEG TPEU remained comparable with only minimal reduction in E and UTS. It was noted, however, that there was a significant increase in the εbreak of the 45CNb-TEG materials attributed to the associative behaviour of the mobile pendant TEG units. Interestingly, it was noted that the 45CNb-MMC polymers exhibited drastic reductions in E, UTS and εbreak owed to the disruptive nature of the bulky pendant groups on the phase separation of the TPEUs (Table 3 and Fig. 4).
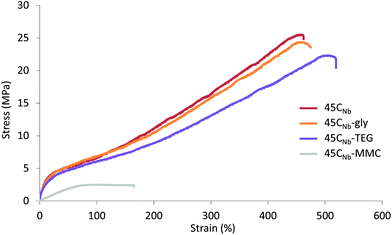 | ||
| Fig. 4 Exemplar stress–strain curves of modified CNb-based TPEUs. Experiments were conducted at ambient temperature (∼25 °C) at an elongation rate of 5 mm min−1 until failure. | ||
Conclusions
In conclusion, pentaerythritol, through the acid catalysed acetal formation, was shown to be an excellent platform in the synthesis of novel functional thermoplastic polyester-urethanes with a range of mechanical properties determined by extender content. Furthermore, as is evident from the study, norbornene-functional TPEUs (CNb) offer a unique route to decorated elastomeric materials which may be employed to control the hydrophilicity and degradation rate of the materials with minimal effect on the mechanical properties through post-polymerisation modifications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Lubrizol Corporation and the University of Warwick are thanked for funding to support the studentship (R. B.).Notes and references
- R. Yoda, J. Biomater. Sci., Polym. Ed., 1998, 9, 561–626 CrossRef CAS.
- A. Lendlein and S. Kelch, Angew. Chem., Int. Ed., 2002, 41, 2034–2057 CrossRef CAS.
- G. Heinrich, M. Klüppel and T. A. Vilgis, Curr. Opin. Solid State Mater. Sci., 2002, 6, 195–203 CrossRef CAS.
- H.-W. Engels, H.-G. Pirkl, R. Albers, R. W. Albach, J. Krause, A. Hoffmann, H. Casselmann and J. Dormish, Angew. Chem., Int. Ed., 2013, 52, 9422–9441 CrossRef CAS PubMed.
- J. Datta and P. Kasprzyk, Polym. Eng. Sci., 2018, 58, E14–E35 CrossRef CAS.
- P. Król, Prog. Mater. Sci., 2007, 52, 915–1015 CrossRef.
- N. Kihara and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 2765–2773 CrossRef CAS.
- A. Lendlein and R. Langer, Science, 2002, 296, 1673 CrossRef PubMed.
- A. Alteheld, Y. Feng, S. Kelch and A. Lendlein, Angew. Chem., Int. Ed., 2005, 44, 1188–1192 CrossRef CAS PubMed.
- Z. Ma, Y. Hong, D. M. Nelson, J. E. Pichamuthu, C. E. Leeson and W. R. Wagner, Biomacromolecules, 2011, 12, 3265–3274 CrossRef CAS PubMed.
- L. Billiet, D. Fournier and F. Du Prez, Polymer, 2009, 50, 3877–3886 CrossRef CAS.
- D. Fournier, B. G. De Geest and F. E. Du Prez, Polymer, 2009, 50, 5362–5367 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- C. J. Hawker and K. L. Wooley, Science, 2005, 309, 1200–1205 CrossRef CAS.
- R. K. Iha, K. L. Wooley, A. M. Nystrom, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS PubMed.
- C. Barner-Kowollik, F. E. Du Prez, P. Espeel, C. J. Hawker, T. Junkers, H. Schlaad and W. Van Camp, Angew. Chem., Int. Ed., 2011, 50, 60–62 CrossRef CAS PubMed.
- I. A. Barker, D. J. Hall, C. F. Hansell, F. E. Du Prez, R. K. O'Reilly and A. P. Dove, Macromol. Rapid. Commun., 2011, 32, 1362–1366 CrossRef CAS.
- C. F. Hansell, P. Espeel, M. M. Stamenović, I. A. Barker, A. P. Dove, F. E. Du Prez and R. K. O'Reilly, J. Am. Chem. Soc., 2011, 133, 13828–13831 CrossRef CAS.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- C. C. Lin, C. S. Ki and H. Shih, J. Appl. Polym. Sci., 2015, 132, 41563 Search PubMed.
- R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 565–598 CrossRef.
- R. J. Williams, I. A. Barker, R. K. O'Reilly and A. P. Dove, ACS Macro Lett., 2012, 1, 1285–1290 CrossRef CAS.
- R. C. Li, R. M. Broyer and H. D. Maynard, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5004–5013 CrossRef CAS.
- E. E. Falco, M. Patel and J. P. Fisher, Pharm. Res., 2008, 25, 2348–2356 CrossRef CAS PubMed.
- A. Hufendiek, S. Lingier and F. E. Du Prez, Polym. Chem., 2019, 10, 9–33 RSC.
- M. C. Arno, R. P. Brannigan, G. M. Policastro, M. L. Becker and A. P. Dove, Biomacromolecules, 2018, 19, 3427–3434 CrossRef PubMed.
- R. P. Brannigan, A. Walder and A. P. Dove, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2279–2286 CrossRef CAS.
- M. J. O'Sickey, B. D. Lawrey and G. L. Wilkes, J. Appl. Polym. Sci., 2002, 84, 229–243 CrossRef.
- R. P. Brannigan, A. Walder and A. P. Dove, Macromolecules, 2016, 49, 2518–2525 CrossRef CAS.
- B. K. Kim, Y. J. Shin, S. M. Cho and H. M. Jeong, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 2652–2657 CrossRef CAS.
- G. Odian, Principles of Polymerization, John Wiley & Sons, Inc., 4th edn, 2004 Search PubMed.
- K. Tashiro and S. Sasaki, Prog. Polym. Sci., 2003, 28, 451–519 CrossRef CAS.
- P. Jaya Ratri and K. Tashiro, Polym. J., 2013, 45, 1019 CrossRef CAS.
- Y. Nozue, Y. Shinohara and Y. Amemiya, Polym. J., 2007, 39, 1221–1237 CrossRef CAS.
- V. Pistor, D. de Conto, F. G. Ornaghi and A. J. Zattera, J. Nanomater., 2012, 2012, 1–8 CrossRef.
- G. Trovati, E. A. Sanches, S. C. Neto, Y. P. Mascarenhas and G. O. Chierice, J. Appl. Polym. Sci., 2010, 115, 263–268 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9py00951e |
| This journal is © The Royal Society of Chemistry 2019 |

