Open Access Article
Open Access ArticleAromatic polymers made by reductive polydehalogenation of oligocyclic monomers as conjugated polymers of intrinsic microporosity (C-PIMs)†
Patrick
Klein
 a,
Hauke J.
Jötten
a,
Catherine M.
Aitchison
a,
Hauke J.
Jötten
a,
Catherine M.
Aitchison
 b,
Rob
Clowes
b,
Eduard
Preis
a,
Andrew I.
Cooper
b,
Rob
Clowes
b,
Eduard
Preis
a,
Andrew I.
Cooper
 b,
Reiner Sebastian
Sprick
b,
Reiner Sebastian
Sprick
 b and
Ullrich
Scherf
b and
Ullrich
Scherf
 *a
*a
aMacromolecular Chemistry, University of Wuppertal, Gaussstrasse 20, 42119 Wuppertal, Germany. E-mail: scherf@uni-wuppertal.de
bMaterials Innovation Factory, University of Liverpool, 51 Oxford Street, Liverpool, L7 3NY, UK
First published on 6th September 2019
Abstract
Reductive dehalogenation polycondensation of a series of penta- or hexacyclic, bisgeminal tetrachlorides with dicobalt octacarbonyl leads to the formation of homopolymers and copolymers with very different optical spectra. While the formation of tetrabenzoheptafulvalene connectors introduces efficient conjugation barriers due to their strongly folded structure, linking of 5-membered ring-based pentacyclic building blocks via bifluorenylidene connectors allows for an extended π-conjugation along the main chain. A comparison of homopolymer P57 and copolymer P55/77 indicates a quite different reactivity for dichloromethylene functions if incorporated into 5- or 7-membered rings. Interestingly, all investigated (co)polymers show an intrinsic microporosity in the solid-state (forming so-called Conjugated Polymers of Intrinsic Microporosity C-PIMs) and have SBET values of up to 760 m2 g−1 for homopolymer P77. This value is one of the highest reported values to date for C-PIMs.
Introduction
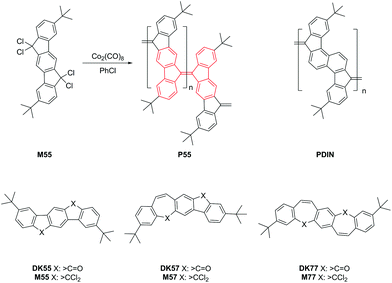
The reductive dehalogenation polycondensation of fully aromatic “bisgeminal tetrachlorides” as reactive diketone derivatives for the synthesis of π-conjugated poly(arylenevinylene)s was pioneered by Hans-Heinrich Hörhold and co-workers in the 1970s1,2 Initially, chromium(II)acetate (Cr2(OAc)4) was used as an efficient dehalogenating reagent in polar solvents such as THF or DMF, or in solvent mixtures containing THF or DMF.1,2 Later, we were successful in generating structurally related, but more highly condensed poly(indenofluorene) PIF/P55 and poly(diindenonaphthalene) PDIN (Scheme 1), whereby the penta- or hexacyclic building blocks are connected via exocyclic double bonds that are made through a reductive polyolefination process; this produces polymers with on-chain bifluorenylidene structural motifs (Scheme 1, the bifluorenylidene unit is labeled in red).3–5 Later, we tested the application of other dehalogenation agents, such as Co2(CO)8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6,7 or Ni(COD)2
6,7 or Ni(COD)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 in chlorobenzene as the suitable non-polar solvent. The products poly(indenofluorene) P55 and poly(diindenonaphthalene) PDIN are low bandgap polyhydrocarbons with long wavelength absorption features ranging into the near infrared (NIR) region. The low bandgap character (Fig. 1), with significantly red-shifted long wavelength absorption bands, was assigned to the contribution of quinoidal resonance states to the electronic ground state. We suggested this was mainly driven by the presence of twisted exocyclic double bonds as result of the crowded steric situation around these double bonds.3,4
8 in chlorobenzene as the suitable non-polar solvent. The products poly(indenofluorene) P55 and poly(diindenonaphthalene) PDIN are low bandgap polyhydrocarbons with long wavelength absorption features ranging into the near infrared (NIR) region. The low bandgap character (Fig. 1), with significantly red-shifted long wavelength absorption bands, was assigned to the contribution of quinoidal resonance states to the electronic ground state. We suggested this was mainly driven by the presence of twisted exocyclic double bonds as result of the crowded steric situation around these double bonds.3,4
 | ||
Fig. 1 UV-Vis spectra of P55 and PDIN in chloroform solutions, λmaxP55: 799 nm (Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), λmaxPDIN: 724 nm (Mn = 30 000 g mol−1), λmaxPDIN: 724 nm (Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), Δλ = 75 nm (0.16 eV); for P55 a LUMO energy level of ca. 3.95 eV and a HOMO energy level of ca. 5.25 eV were estimated, from ref. 3 and 4. 000 g mol−1), Δλ = 75 nm (0.16 eV); for P55 a LUMO energy level of ca. 3.95 eV and a HOMO energy level of ca. 5.25 eV were estimated, from ref. 3 and 4. | ||
Results and discussion
In this work, we develop related pentacyclic tetrachloro-monomers that contain 7-membered (cycloheptatriene) instead of the initially used 5-membered (cyclopentadiene) connector rings by introducing one or two additional vinylene groups into the monomers (Scheme 1). With this set of three monomers (3,9-di-tert-butyl-6,6,12,12-tetrachloro-6,12-dihydroindeno[1,2-b]fluorene M55: the already tested indenofluorene-based monomer for P55 synthesis, 2,8-di-tert-butyl-6,6,14,14-tetrachloro-6,14-dihydrobenzo[4,5]cyclohepta[1,2-b]fluorene M57, and 3,11-di-tert-butyl-5,5′,13,13′-tetrachlor-dibenzo[d,d′]benzo[1,2-a:4,5-a′]di[7]annulene M77) we have carried out a series of reductive dehalogenation polycondensation reactions, including that of the known homopolymer P55 – the already well-known poly(indenofluorene) PIF, and of the new homopolymers P57 (resulting from polycondensation of the AB-type monomer M57) and P77 (resulting from polycondensation of monomer M77) as well as a copolymer P55/77 resulting from polycondensation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of monomers M55 and M77 (in a AA/BB copolymerization scheme).
1 molar mixture of monomers M55 and M77 (in a AA/BB copolymerization scheme).
First, we studied the homopolycondensation of monomer M77 yielding P77: as a corresponding dimeric model compound – tetrabenzoheptafulvalene (TBHF) (Scheme 2, red labeled part) – it is well known that a strongly folded, anti- or syn-type isomeric arrangement of the two non-planar, boat-shaped dibenzannelated 7-membered cycloheptatriene rings is adopted with nearly no electronic (conjugative) interaction across the exocyclic double bond. This was first derived from a NMR-spectroscopic analysis, and later complemented by single crystal structure analysis of both isomers.9–12 This arrangement is in strong contrast to the electronic properties of the tetrabenzopentafulvalene (bifluorenylidene, TBPF) system with its ca. 42° twisted exocyclic double bond that connects two, almost planar fluorenylidene units.13,14 Next, we tested the polycondensation of the “mixed” monomer M57 that contains one 5- and 7-membered ring each15 and, for comparison, of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M55/M77 monomer mixture. Since we expect a quite different reactivity of both geminal >CCl2-functions in the reductive polyolefination when incorporated into 5- or 7-membered rings, we investigated the incorporation pattern of the monomeric subunits into the resulting fulvalene linkages in more detail. In particular, we compared chemical structure and optical/electronic properties of homopolymer P57 and copolymer P55/77.
1 M55/M77 monomer mixture. Since we expect a quite different reactivity of both geminal >CCl2-functions in the reductive polyolefination when incorporated into 5- or 7-membered rings, we investigated the incorporation pattern of the monomeric subunits into the resulting fulvalene linkages in more detail. In particular, we compared chemical structure and optical/electronic properties of homopolymer P57 and copolymer P55/77.
 | ||
| Scheme 2 Syntheses scheme for monomers M57 and M77 as well as homopolymers P77 and P57, and copolymer P55/77. | ||
Monomer and polymer synthesis
As mentioned above, reductive polycondensation of the tetrachloro monomers M77 and M57 gives homopolymers P77 and P57, respectively. The non-alkylated analogues of the diketone model compounds DK77 and DK57 were already described in the literature.16,17 Instead of following the published route for non-alkylated DK77, we adapted a more recent route published by Yang et al.15 for DK57, starting for both monomers from 2,5-dibromoterephthalic acid, which is a frequently used starting material for ladder-type polyarylenes made by us previously.18,19For the synthesis of M57 and M77, we used an elegant procedure by Ciganek et al. involving a one-pot dehydrogenation/chlorination of the corresponding, single- or double-ethane-bridged diketones 5.20 In contrast to the corresponding five-ring or acyclic tetrachloro derivatives,3,4,6,7 the bisgeminal tetrachlorides containing seven-membered rings are much more air sensitive: simple recrystallisation from n-hexane or toluene under air yielded the unsaturated diketone derivatives, indicated by the occurrence of keto carbon resonances at around 190 ppm in the 13C{1H} NMR spectra. Therefore, after removal of excess POCl3/toluene and PCl5 by vacuum distillation and sublimation, the air sensitive compounds M57 and M77 were directly used in the subsequent polycondensation reactions with dicobalt octacarbonyl in chlorobenzene without further purification to give P57 and P77, respectively. P55/77 was obtained by chlorination of 5a and 5b to give M55 and M77, respectively, followed by subsequent polycondensation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer mixture.
1 monomer mixture.
Homopolymer P77
Polymer P77 was obtained as yellow powder after work-up and solvent fractionation using Soxhlet extraction, with molecular weight (MW) averages Mn of 9500 (DP: ca. 22) for the minority ethyl acetate fraction (16%), and Mn of 4550 (DP: ca. 10) for the main, lower molecular weight acetone fraction (81%) thus confirming the successful polycondensation up to the polymer limit despite the highly sterically demanding environment around the coupling positions. In the 13C{1H} NMR spectrum of P77, the two aliphatic carbon signals of the tert-butyl side groups are doubled, probably indicating the presence of both cis- (Z-) and trans- (E-) isomeric arrangements of the pentacyclic repeating units with respect to the exocyclic double bonds (Scheme 3 depicts the trans configuration). The aromatic 13C NMR signals of P77 are distinctly broadened as consequence of the polymer formation (for 1H and 13C{1H} NMR spectra see Fig. S13 in the ESI†).Fig. 2 shows the optical spectra of P77 (higher MW ethyl acetate fraction) and the corresponding diketone precursor DK77 in diluted chloroform solution. The absorption range is restricted to the UV region due to the strongly folded structure of the polymer. The diketone DK77 shows a low intensity long wavelength n–π* transition (as shoulder at ca. 430 nm) and a higher intensity π–π* transition peaking at ca. 361 nm. In the polymer, the π–π* transition-related band is blue-shifted to 331 nm by Δλ = 30 nm, thus reflecting the presence of localised, isolated chromophores with the electronic interaction restricted to one cis-distyrylbenzene unit, and without noticeable conjugation across the folded exocyclic double bond. The photoluminescence spectra of precursor diketone DK77 and polymer P77 display emission maxima (0-0 transitions) at λmax (PL) of 492 nm for the diketone and a hypsochromically shifted deep blue PL peaking at 419 nm for the polymer P77 (Δλ = 73 nm, photoluminescence quantum yield PLQY of P77 in chloroform solution: 2.54%). The absorption spectrum of P77 as a thin-film (see Fig. S24 in the ESI†) only shows a low intensity shoulder in the region of the long wavelength absorption band of the chloroform solution (ca. 340 nm), without any longer wavelength absorption features.
 | ||
| Fig. 2 Absorption and photoluminescence spectra of P77 and the corresponding diketone precursor DK77 (solvent: chloroform, excitation wavelength λexc: 310 nm for P77, and 330 nm for DK77. | ||
Homopolymer P57 and Copolymer P55/77
The synthesis of a non-alkylated analogue of M57 was already described in the literature.13 The alkylated analogue was generated as depicted in Scheme 2, the finally prepared bisgeminal tetrachloride M57 was obtained by reaction of the diketone precursor with phosphorous pentachloride. Due to its air sensitivity the monomer was directly used in a reductive olefination reaction with dicobalt octacarbonyl. After work-up and solvent fractionation, P57 was obtained as red powder with Mn: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (DP: ca. 26) for the ethyl acetate fraction (33%), while the lower molecular weight acetone fraction (51%) showed a molecular weight Mn of 3600 (DP: ca. 9). In contrast to P77, in the 13C{1H} NMR spectrum of P57, the aliphatic signals are not clearly doubled which may be a result of the asymmetry of the repeat unit (for the related 1H and 13C{1H} NMR spectrum of the diketone DK57 see Fig. S12 in the ESI†).
400 (DP: ca. 26) for the ethyl acetate fraction (33%), while the lower molecular weight acetone fraction (51%) showed a molecular weight Mn of 3600 (DP: ca. 9). In contrast to P77, in the 13C{1H} NMR spectrum of P57, the aliphatic signals are not clearly doubled which may be a result of the asymmetry of the repeat unit (for the related 1H and 13C{1H} NMR spectrum of the diketone DK57 see Fig. S12 in the ESI†).
P55/77 was obtained in a random copolymerisation of monomers M55 and M77. Hereby, equimolar amounts of both diketone precursors were chlorinated. After removal of solvents/POCl3 and sublimation of excess PCl5, both products were dissolved in chlorobenzene and reacted in a reductive co-polycondensation with dicobalt octacarbonyl. After work-up and solvent fractionation, the product P55/77 was obtained as a deep blue powder, with a Mn of 5500 (DP: ca. 14) for the ethyl acetate fraction (13%), while the lower molecular weight acetone fraction (21%) has a Mn of 2900 (DP: ca. 7).
The very similar positions of the absorption maxima of P57 at 485 nm and the isolated TBPF chromophore (λmax: 460 nm, see Fig. 3)21 indicates the dominating presence of alternating twisted TBPF and folded TBHF motifs in P57 as consequence of a very different reactivity of the two different dichloromethylene functions of M57 during the reductive polycondensation, with the higher reactivity most probably for the sterically less stressed –CCl2– units incorporated into 5-membered rings. Hereby, the TBHF building blocks formed in the second condensation step act as efficient conjugation barriers and lead to the presence of isolated TBPF chromophores within the polymer main chain that is composed in an alternating order of TBHF and TBPF units. Moreover, the distinctly red-shifted absorption band of P55/77 peaking at 705 nm, if compared to P57 (486 nm), indicates the presence of more extended, π-conjugated oligoindenofluorene segments (for a study of the absorption properties of M55-derived oligomers see ref. 22) as consequence of the preferred coupling of the sterically less demanding dichloromethylene functions of M55, thus resulting in a tendency for (multi)block copolymer formation. Similar results have been described for the Yamamoto-type polycondensation of two different dibromoarylene/dibromoheteroarylene monomers.23
Gas sorption
Linear and soluble polymers of intrinsic microporosity (so-called PIMs) have been known since the pioneering work of Budd, McKeown and co-workers.25,26 Several folding motifs, including on-chain spirobifluorene27,28/spirobiindane,29 binaphthyl,30 triptycene31 or norbornyl bis(benzocyclobutene)32 units, have been introduced to give a shape-persistent folding of rigid-rod type polycyclic segments of linear polymer chains that pack into bulk materials with a persistent free volume.33 Also several conjugated polymers (CPs) with bulky main chain substituents, including poly(trimethylsilylacetylene)s34 or poly(diphenylacetylene)s35 and CPs containing 1,3-phenylene units36–39 show a permanent microporosity with SBET surface areas up to 730 m2 g−1 (so-called conjugated PIMs—C-PIMs). Based on these concepts we have tested if the incorporation of strongly folded tetrabenzoheptafulvalene (TBHF) or twisted bifluorenylidene motifs into the linear, polycyclic homopolymers P55, PDIN, P77 and P57 as well as the copolymer P55/77 also induces a permanent microporosity. For the already known P55 and PDIN, we expected the materials to be microporous due to the twisted bifluorenylidene motifs, which is similar to the orthogonal spirobisindane motif in PIM-1.24The microporosity properties of the polymers P55, PDIN, P57, P77 and P55/77 were studied by nitrogen gas adsorption/desorption measurements at 77 K. All polymers showed typical type-I isotherms with a significant amount of nitrogen adsorption at low pressure, indicating presence of micropores, a slowly increasing gas adsorption in the mid pressure range, followed by increased adsorption at higher relative pressure (P/P0 > 0.9) especially for PDIN, probably caused by multilayer formation in meso- or macropores (see Fig. S17 in the ESI†). Differential pore size distributions obtained by application of hybrid density functional theory confirmed the mostly microporous nature of the polymers, with the maximum values in the range of 1–2 nm (see Fig. S20 in the ESI†). We observed a pronounced hysteresis between adsorption and desorption curves, which may either be attributed to pore network effects and various forms of pore blocking with capillary condensation or to swelling effects that were also observed for other PIMs and flexible, amorphous networks.24,40–43 PXRD patterns confirmed the amorphous nature for all polymers discussed here (see Fig. S22 in the ESI†).
The estimated surface areas for the polymers ranged from 543 m2 g−1 for P55/77 to 757 m2 g−1 for P77 (see Table 1), comparing well with the currently highest reported values for C-PIMs (see Table S1 in the ESI† for a comparative listing of literature values). Interestingly, a drop-casted thin film of P55 maintained its microporosity to nitrogen and showed a SBET surface area of 333 m2 g−1, thus demonstrating strong potential for future practical applications. Considering the strong influence of processing conditions on the apparent surface area of soluble porous polymers, we tested additionally washing a freshly precipitated powder sample with supercritical carbon dioxide (scCO2), which proved to strongly affect the SBET surface area in the past.44 The SBET surface area only slightly increased when a powder sample of P55 was additionally purified by supercritical carbon dioxide scCO2 washing (738 vs. 687 m2 g−1) thus confirming a reliable sample preparation protocol. Sorption isotherms as well as SEM images of powder and film indicated very different morphologies with a substantial loss of meso- and macroporosity for the film, probably causing the decreased overall SBET surface area (see Fig. S23 in the ESI†).
| Polymer | M n (kDa) | M w (kDa) | M w/Mn | DP | λ max (nm) | S BET (m2 g−1) | V total (cm3 g−1) | V micro (cm3 g−1) | H2 uptake (77 K)a (mmol g−1) | CO2 uptake (273 K)a (mmol g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Uptake at 1 bar. b Powder sample with a Mn: 13.2 kDa, Mw: 20.7 kDa. c Powder sample with a Mn: 7.2 kDa; Mw: 13.9 kDa. d After scCO2 washing of a powder sample with Mn: 7.2 kDa; Mw: 13.9 kDa. e After drop-casting the sample from chloroform solution. f Powder sample with Mn: 5.3 kDa; Mw: 11.0 kDa. | ||||||||||
| P77 | 9.5 | 20.2 | 2.1 | 23 | 331 | 757b | 0.58 | 0.22 | 5.1 | 1.8 |
| P57 | 10.4 | 14.4 | 1.4 | 26 | 485, 321 | 609 | 0.39 | 0.17 | 4.3 | 1.6 |
| P55/77 | 5.5 | 7.4 | 1.4 | 14 | 705, 309 | 543 | 0.41 | 0.15 | 3.6 | 1.2 |
| P55 (PIF) | 17.0 | 40.0 | 2.4 | 47 | 799, 309 | 687c (738d; 333e) | 0.51 | 0.18 | 4.6 | 1.6 |
| PDIN | 25.0 | 57.0 | 2.3 | 61 | 724, 324 | 691f | 0.68 | 0.16 | 4.1 | 1.4 |
To explore the potential for gas storage- or separation, we conducted sorption measurements using carbon dioxide (at 273 K) and hydrogen (at 77 K) (see Fig. S19 in the ESI†). Interestingly, P57 showed a higher uptake for carbon dioxide and hydrogen than PDIN. A possible explanation might be that micropores of P57 may be inaccessible for N2, but accessible to CO2 and H2. The highest uptakes (see Table 1) of 1.8 mmol g−1 CO2 and 5.1 mmol g−1 H2 at ca. 1 bar for P77, comparing well with other highly porous C-PIMs.37
Conclusions
In summary, we present the synthesis of three novel homo- and copolymers P77, P57 and P55/77, where pentacyclic building blocks are linked together by exocyclic double bonds, in addition to the already known P55 and PDIN. The (co)polymers are made by reductive dehalogenation polycondensation of pentacyclic, bisgeminal tetrachlorides with dicobalt octacarbonyl, a protocol that was established by our group. All monomers contain reactive dichloromethylene functions that are incorporated into 5- or 7-membered rings. The results presented here indicate an interrupted conjugation along exocyclic double bonds for the tetrabenzoheptafulvalene (TBHF) connector, while tetrabenzopentafulvalene (TBPF) connectors allow for a main chain π-electron delocalization. This leads to quite different optical (absorption) spectra of the polymers, for the polymer P77 containing exclusively TBHF connections the absorption range is restricted to the UV area. By introducing TBPF motifs into the polymer backbone, the long wavelength absorption band is gradually red-shifted in parallel with the length of the present conjugated segments. These results also indicate a higher reactivity of the bisgeminal dichlorides that are incorporated into 5-membered rings, supported by the obvious formation of multiblock copolymers for M55/M77 monomer mixtures and by the formation of alternating TBHF and TBPF connections during coupling of M57 into P57.All (co)polymers are microporous and constitute new examples of the relatively uncommon sub-class of materials known as ‘Conjugated Polymers of Intrinsic Microporosity’ (C-PIMs). BET surface areas are as high as 757 m2 g−1. All (co)polymers showed SBET surface areas in a narrow range between 500 to 800 m2 g−1. These results impressively demonstrate that both non-planar, twisted tetrabenzopentafulvalene (TBPF, bifluorenylidene) as well as folded tetrabenzoheptafulvalene (TBHF) motifs both induce a significant intrinsic microporosity of the linear C-PIMs in the solid state, thus representing very promising building blocks in the development of functional C-PIMs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Sylwia Adamczyk and Dr. Ammar H. Alahmed for help with gas sorption, Anke Helfer for the GPC, and Andreas Siebert for NMR measurements. We thank the Engineering and Physical Sciences Research Council (EPSRC) for financial support under Grant EP/N004884/1.Notes and references
- H.-H. Hörhold, J. Gottschaldt and J. Opfermann, J. Prakt. Chem., 1977, 319, 611 CrossRef.
- H.-H. Hörhold and D. Raabe, Acta Polym., 1979, 30, 86 CrossRef.
- H. Reisch, U. Wiesler, U. Scherf and N. Tuytuylkov, Macromolecules, 1996, 29, 8204 CrossRef CAS.
- E. Preis and U. Scherf, Macromol. Rapid Commun., 2006, 27, 1105 CrossRef CAS.
- E. J. Meijer, D. M. de Leeuw, S. Setayesh, E. van Veenendaal, B.-H. Huisman, P. W. M. Blom, J. C. Hummelen, U. Scherf and T. M. Klapwijk, Nat. Mater., 2003, 2, 678 CrossRef CAS.
- E. Preis, W. Dong, G. Brunklaus and U. Scherf, J. Mater. Chem. C, 2015, 3, 1582 RSC.
- S. Baysec, E. Preis and U. Scherf, Macromol. Rapid. Commun., 2016, 37, 1802 CrossRef CAS.
- H. Reisch, Oligo- und Poly(indenofluorene) – neue Materialien mit einer kleinen Bandlücke, PhD Thesis, University of Mainz, Germany, 2000 Search PubMed.
- W. Treibs and H.-J. Klinkhammer, Chem. Ber., 1951, 84, 671 CrossRef CAS.
- A. Schönberg, U. Sodke and K. Praefcke, Chem. Ber., 1969, 102, 1453 CrossRef.
- K. S. Dichmann, S. C. Nyburg, F. H. Pickard and J. A. Potworowski, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 27 CrossRef CAS.
- J. Luo, K. Song, F. I. Gu and Q. Miao, Chem. Sci., 2011, 2, 2029 RSC.
- A. Takai, D. J. Frea, T. Suzuki, M. Sugimoto, J. Labuta, R. Haruki, R. Kumai, S.-I. Adachi, H. Sakai, T. Hasobe, Y. Matsushita and M. Takeuchi, Org. Chem. Front., 2017, 4, 650 RSC.
- P. U. Biedermann, J. J. Stezowski and I. Agranat, Eur. J. Org. Chem., 2001, 15 CrossRef CAS.
- X. Yang, X. Shi, N. Aratani, T. P. Goncalves, K.-W. Huang, H. Yamada, C. Chi and Q. Miao, Chem. Sci., 2016, 7, 6176 RSC.
- I. Agranat and D. Avnir, J. Chem. Soc., Perkin Trans. 1, 1974, 1155 RSC.
- X. Yang, D. Liu and Q. Miao, Angew. Chem., Int. Ed., 2014, 53, 6786 CrossRef CAS.
- K.-J. Kass, M. Forster and U. Scherf, Angew. Chem., Int. Ed., 2016, 55, 7816 CrossRef CAS.
- M.-C. Ockfen, M. Forster and U. Scherf, Macromol. Rapid Commun., 2018, 1800569 CrossRef PubMed.
- E. Ciganek, R. T. Uyeda, M. Cohen and D. H. Smith, J. Med. Chem., 1981, 24, 336 CrossRef CAS.
- F. Straus, R. Kühnel and R. Hänsel, Ber. Dtsch. Chem. Ges. A/B, 1933, 66, 1847 CrossRef.
- H. Reisch and U. Scherf, Synth. Met., 1999, 101, 128 CrossRef CAS.
- U. Asawapirom and U. Scherf, Macromol. Rapid Commun., 2001, 22, 746 CrossRef CAS.
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 2, 230 RSC.
- P. M. Budd, E. S. Elabas, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib, C. E. Tattershall and D. Wang, Adv. Mater., 2004, 16, 456 CrossRef CAS.
- P. M. Budd, K. J. Msayib, C. E. Tattershall, B. S. Ghanem, K. J. Reynolds, N. B. McKeown and D. Fritsch, J. Membr. Sci., 2005, 251, 263 CrossRef CAS.
- J. Weber, Q. Su, M. Antonietti and A. Thomas, Macromol. Rapid Commun., 2007, 28, 1871 CrossRef CAS.
- D. Becker, N. Konnertz, M. Böhning, J. Schmidt and A. Thomas, Chem. Mater., 2016, 28, 8523 CrossRef CAS.
- P. M. Budd, N. B. McKeown and D. Fritsch, Macromol. Symp., 2006, 245, 403 CrossRef.
- N. Ritter, M. Antonietti, A. Thomas, I. Senkovska, S. Kaskel and J. Weber, Macromolecules, 2009, 42, 8017 CrossRef CAS.
- B. S. Ghanem, M. Hashem, K. D. M. Harris, K. J. Msayib, M. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43, 5287 CrossRef CAS.
- H. W. H. Lai, Y. Chin and Y. Xia, ACS Macro Lett., 2017, 6, 1357 CrossRef CAS.
- P. M. Budd and N. B. McKeown, Chem. Soc. Rev., 2006, 35, 675 RSC.
- T. Masuda, E. Isobe, T. Higashimura and K. Takada, J. Am. Chem. Soc., 1983, 105, 7473 CrossRef CAS.
- W.-E. Lee, D.-C. Han, D.-H. Han, H.-J. Choi, T. Sakaguchi, C.-L. Lee and G. Kwak, Macromol. Rapid Commun., 2011, 32, 1047 CrossRef CAS PubMed.
- G. Cheng, T. Hasell, A. Trewin, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2012, 124, 12899 CrossRef.
- G. Cheng, B. Bonillo, R. S. Sprick, D. J. Adams, T. Hasell and A. I. Cooper, Adv. Funct. Mater., 2014, 24, 5219 CrossRef CAS.
- R. S. Sprick, J.-X. Jiang, B. Bonillo, S. Ren, T. Ratvijitvech, P. Guiglion, M. A. Zwijnenburg, D. J. Adams and A. I. Cooper, J. Am. Chem. Soc., 2015, 137, 3265 CrossRef CAS.
- R. S. Sprick, Y. Bai, A. A. Y. Guilbert, M. Zbiri, C. M. Aitchison, L. Wilbraham, Y. Yan, D. J. Woods, M. A. Zwijnenburg and A. I. Cooper, Chem. Mater., 2019, 31, 305 CrossRef CAS.
- S. Qiao, Z. Du and R. Yang, J. Mater. Chem. A, 2014, 2, 1877 RSC.
- J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334 CrossRef CAS.
- N. B. McKeown, S. Hanif, K. Msayib, C. E. Tattershall and P. M. Budd, Chem. Commun., 2002, 23, 2782 RSC.
- J. Jeromenok and J. Weber, Langmuir, 2013, 29, 12982 CrossRef CAS.
- E. Preis, C. Widling, U. Scherf, S. Patil, G. Brunklaus, J. Schmidt and A. Thomas, Polym. Chem., 2011, 2, 2186 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Monomer and polymer synthetic procedures for new compounds, NMR data, optical spectra, PXRD patterns for polymers, gas sorption data, TGA data, SEM images. See DOI: 10.1039/c9py00869a |
| This journal is © The Royal Society of Chemistry 2019 |