Chiral helical substituted polyacetylene grafted on hollow polymer particles: preparation and enantioselective adsorption towards cinchona alkaloids†
Abstract
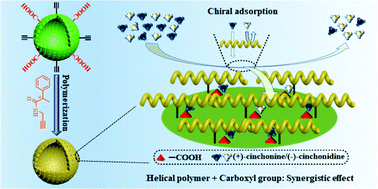
Incorporating chiral helical structures with porous architectures and/or functionalities in a single entity is a promising strategy, which may provide various advanced functional materials with potential applications that cannot be achieved in routine materials. In this contribution, hollow polymer particles (HPPs) were constructed by biomass trans-anethole and maleic anhydride and employed as a unique support for grafting chiral helical substituted polyacetylene. To prepare the designed chiral hollow particles, HPPs were firstly reacted with propargylamine to form HPPs simultaneously bearing carboxyl groups and polymerizable alkynyl groups on the shell (the particles were named M-HPPs). After copolymerizing the alkynyl groups tethered on M-HPPs with a chiral acetylenic monomer (M1, R or S), hollow particles grafted with chiral helical polymer chains were obtained. The resulting hollow particles exhibit fascinating optical activity and enantioselective adsorption capacity towards cinchona alkaloids, in which (+)-cinchonine/(−)-cinchonidine were used as chiral drug models. Taking advantage of the preparation strategy, a number of novel chiral hollow particles of the kind may be constructed next. They are anticipated to find wide applications in chiral-related areas.


 Please wait while we load your content...
Please wait while we load your content...