Hydrolytically-degradable homo- and copolymers of a strained exocyclic hemiacetal ester†
Abstract
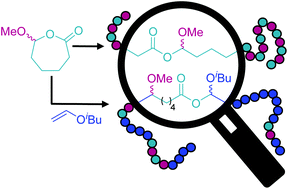
We report the cationic ring-opening homo- and copolymerization of the 7-membered exocyclic hemiacetal ester 7-methoxyoxepan-2-one (MOPO) to afford poly(7-methoxyoxepan-2-one) [poly(MOPO)] and its copolymers with isobutyl vinyl ether (IBVE) that achieve essentially quantitative conversions of MOPO. The amorphous homopolymer is characterized by a low glass transition temperature of Tg = −37 °C and is readily degraded under both acidic and basic conditions at the labile acylacetal linkages. With triflic acid as the cationic initiator the homopolymerization proceeds by an uncontrolled active chain end mechanism to reproducibly yield poly(MOPO) of Mn ∼ 20 kg mol−1. The monomer was also polymerized by a photoinitiated process using a dithiocarbamate or trithiocarbonate chain transfer agent and the photocatalyst 2,4,6-tris(p-4-methoxyphenyl)pyrylium tetrafluoroborate. Lower molar mass polymers were recovered at high initial concentrations of dithiocarbamate as compared to no chain transfer agent, indicating some ability to control polymer molar mass. Photoinitiated copolymerization of MOPO and IBVE afforded a tapered copolymer with MOPO-MOPO, IBVE-MOPO, and IBVE-IBVE linkages that degraded into low molar mass poly(IBVE) fragments in hydrochloric acid through hydrolysis of the acylacetal linkages resulting from ring-opened MOPO units in the backbone.
- This article is part of the themed collection: Polymer Chemistry Lectureship Winners


 Please wait while we load your content...
Please wait while we load your content...
