Selective or living organopolymerization of a six-five bicyclic lactone to produce fully recyclable polyesters†
Abstract
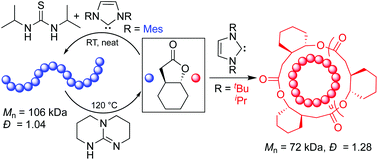
Organocatalyzed ring-opening polymerization (O-ROP) of a six-five bicyclic lactone, 4,5-trans-cyclohexyl-fused γ-butyrolactone (4,5-T6GBL), can be topologically selective or living at room temperature, depending on catalyst structure. A screening of (thio)urea [(T)U] and organic base pairs revealed unique trends in reactivity for this monomer as well as the most active catalyst pairs, which were employed as received commercially to produce relatively high molecular weight (Mn up to 106 kDa), low dispersity (Đ = 1.04) linear poly(4,5-T6GBL) in a living fashion. The ROP using a hybrid organic/inorganic pair of TU/KOMe in neat conditions led to poly(4,5-T6GBL) with even higher molecular weight (Mn = 215 kDa, Đ = 1.04). In comparison to the metal-catalyzed system, (T)U-base pairs exhibited competitive kinetics and reached higher monomer conversions, and their reactions can be performed in air. In addition, the resulting polymers required less purification to produce materials with higher onset decomposition temperature. (T)U-base pairs were selective towards linear polymerization only, whereas triazabicyclodecene can catalyze both polymerization and (quantitative) depolymerization processes, depending on reaction conditions. Cyclic polymers with Mn = 41–72 kDa were selectively formed via N-heterocyclic carbene-mediated zwitterionic O-ROP.
- This article is part of the themed collection: Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...
