Acidity-triggered zwitterionic prodrug nano-carriers with AIE properties and amplification of oxidative stress for mitochondria-targeted cancer theranostics†
Abstract
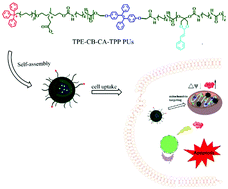
Although biodegradable polymer–drug conjugate delivery systems exhibit great promise toward many types of cancers, several challenges still remain, associated with specific targeting of organelles as well as real-time tracking and observing. Herein, an innovative pH-responsive zwitterionic prodrug is prepared that can easily self-assemble into stable micelles with high drug loading. This new nanocarrier is composed of the following vital factors: (i) a zwitterionic outer shell to prolong the blood circulation and aggregate at the tumor site; (ii) surface-encoded triphenyl-phosphonium groups to enhance organelle targeting; (iii) ketal bonds of nanomicelles to realize the controlled release of therapeutic molecules; (iv) aggregation-induced emission (AIE) luminogen tetraphenylethene (TPE) to realize labelling and real-time monitoring of the mitochondria in living cells. This targeted pH-responsive polyprodrug can effectively release therapeutic molecules to activate the generation of the local ROS level and enable real-time monitoring of the status of mitochondria, which presents a practicable strategy for simultaneous cancer diagnostics and therapeutics.


 Please wait while we load your content...
Please wait while we load your content...