Self-assembly of highly asymmetric, poly(ionic liquid)-rich diblock copolymers and the effects of simple structural modification on phase behaviour†
Abstract
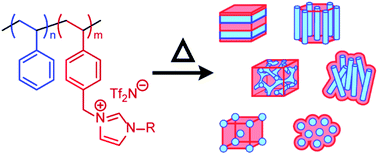
A series of thermally processable, phase-separating diblock copolymers made via sequential ATRP of styrene and styrenic ionic liquid (IL) monomers with various alkyl imidazolium substituents were synthesized to cover a wide range of volume fractions, most notably those on the IL-rich side of the phase diagram. Small-angle X-ray scattering (SAXS) analysis was used to confirm melt-state (and glassy state) phase behavior in which all four classic equilibrium diblock copolymer morphologies – body-centered cubic spheres (SBCC), hexagonally packed cylinders (Hex), lamellae (Lam), and notably, bicontinuous gyroid (Gyr) – were observed. These PS-PIL diblock copolymers were found to have a high degree of conformational asymmetry and/or electrostatic cohesion within the PIL block, highlighted by the shift of the Lam phase window with boundaries falling between fPIL = 0.31 and 0.55. Variation of the alkyl group appeared to influence the strength of the Flory-like interaction parameter of the system, χPS/PIL, such that simple substitution of methyl by n-butyl on the imidazolium IL substituent resulted in the emergence of the (notoriously segregation-sensitive) Gyr phase, superseding the persistent coexistence of Lam and Hex in the methyl-substituted imidazolium diblock copolymer phase diagram.


 Please wait while we load your content...
Please wait while we load your content...