Regioselective post-functionalization of isotactic polypropylene by amination in the presence of N-hydroxyphthalimide†
Abstract
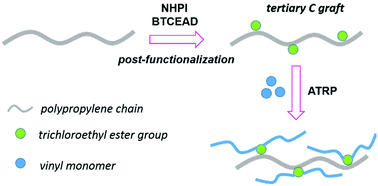
Previously, we developed amination modification of polyethylene by metal-free organocatalytic reaction. Here, we applied this chemistry to modify isotactic polypropylene (PP) to investigate the regioselectivity to different carbons. Specifically, this reaction was conducted in tetrachloroethane at 100 °C under a nitrogen protection where C–N bonds were selectively generated from the tertiary C–H bonds of the PP backbone in the presence of N-hydroxyphthalimide (NHPI) and bis(2,2,2-trichloroethyl) azodicarboxylate (BTCEAD). The grafting content of BTCEAD was as high as 4.7 units per 100 propylene units. Thus, a methodology for the regioselective post-functionalization of PP without metal catalysts has been developed. The obtained PP-g-BTCEAD was sequentially used as an atom transfer radical polymerization (ATRP) macro-initiator to graft polymers, such as polystyrene (PS) and poly(methyl) methacrylate (PMMA). The grafting polymerization was unequivocally proved by multiple characterization methods. The graft copolymers were applied as phase compatibilizers to improve blending of PP with PS and PMMA.


 Please wait while we load your content...
Please wait while we load your content...