Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Trends in polymeric shape memory hydrogels and hydrogel actuators
Jiaojiao
Shang
 a,
Xiaoxia
Le
b,
Jiawei
Zhang
a,
Xiaoxia
Le
b,
Jiawei
Zhang
 b,
Tao
Chen
b,
Tao
Chen
 *b and
Patrick
Theato
*b and
Patrick
Theato
 *cd
*cd
aInstitute for Technical and Macromolecular Chemistry, University of Hamburg, Bundesstrasse 45, D-20146 Hamburg, Germany
bDepartment of Polymers and Composites, Key Laboratory of Bio-based Polymeric Materials Technology and Application of Zhejiang Province, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, 1219 Zhongguan West Road, 315201 Ningbo, Zhejiang, China
cInstitute for Chemical Technology and Polymer Chemistry, Karlsruhe Institute of Technology (KIT), Engesser Str. 18, D-76131 Karlsruhe, Germany
dInstitute for Biological Interfaces III, Karlsruhe Institute of Technology (KIT), Herrmann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany. E-mail: tao.chen@nimte.ac.cn; patrick.theato@kit.edu
First published on 17th January 2019
Abstract
Recently, “smart” hydrogels with either shape memory behavior or reversible actuation have received particular attention and have been further developed into sensors, actuators, or artificial muscles. These three-dimensional polymer networks with the capability of shape deformations show a volume phase transition after being triggered by external physicochemical stimuli. Here, we review the recent advancements and the different types of shape memory hydrogels (SMHs). In addition, stimuli-responsive hydrogel actuators have been investigated with a special focus on their stimulation, their motion-deformation strategies and on the fabrication technologies adopted in hydrogel-based actuators, and finally their applications are described and discussed using specific examples.
1. Introduction
Hydrogels are water-swollen three-dimensional polymeric networks that have been investigated by researchers due to their exceptionally promising potential in a wide range of applications, such as drug delivery,1–3 sealing,4–6 tissue engineering,7–9 actuators,10–12etc. Compared to the corresponding bulk polymer films, hydrogels with a large amount of water are soft and flexible enough to enable deformations. Additionally, the highly open structure, the large inner surface, and the 3D network of hydrogels are desirable for bioapplications as biosensors and actuators.13 Particularly, stimuli-responsive behaviors inspired by nature, such as the opening and closing of flowers and the release of seeds from pine cones are affected by light or temperature, and are among the most attractive features of hydrogels that have gained special attention. Generally, hydrogels with “smart” behaviors undergo reversible shape deformations in response to external stimuli, such as heat, light, or electricity. Nowadays, as two of the most famous “smart” hydrogels, shape memory hydrogels (SMHs) and hydrogel actuators (HAs) have attracted increasing attention due to their promising potential applications in biomedicine14 and soft robotics,15 and as artificial muscles16 or as micro-swimmers.17SMHs, as the name implies, are able to fix temporary shapes and recover to their original shape by forming or destructing reversible crosslinks upon external stimuli (e.g. by heat, magnetism, light and chemicals). Usually, during this process, some networks of SMHs maintain their permanent shape, and dynamic networks are applied for shape memory.18,19 Up to now, there have been three main trends in SMH research, such as tough SMHs, triple-/multi-SMHs and multifunctional SMHs.20,21 Due to the introduction of reversible interactions into hydrogel systems for achieving a shape memory behavior, SMHs tend to have weak mechanical properties. As a result, various approaches, including constructing double networks, dual/triple crosslinks in a single network, have been developed for fabricating tough SMHs. Since the number of temporary shapes can have an effect on promising applications, SMHs with a triple-/multi-shape memory effect have attracted broad attention. What's more, SMHs with a single function can no longer meet the request for wider potential applications. Hence, functions such as self-healing behavior, thermoplasticity, and adhesion properties have already been integrated into SMHs.22–25 Different from SMHs, without a bonding/bond-breaking process, HAs’ actuation performance is due to asymmetric swelling. HAs are based on stimuli-responsive synthetic polymers that are able to swell and shrink in water in response to the changes of environmental stimuli. Since the volume phase transition was first referred to by Tanaka,26 many hydrogel actuators have been developed as soft actuators due to their flexible and soft characteristics, but also using mimetic motion without the use of mechanical devices, generated from the volume phase transition of crosslinked polymer hydrogels. For HAs, the structures of designed hydrogels determine their response time, application, sensing, actuation and shape changes. Furthermore, the deformation of hydrogels depends mainly on their inhomogeneous structures. Hence, the design principles of hydrogel actuators based on inhomogeneous structures have been described in many reports.27–29 Nowadays, thanks to the rapid development of microtechnology, technologies for fabricating and designing subtle actuators, which meet the necessity of soft actuators used in microscales, have achieved promising growth. In this aspect, photo-lithography and 3D printing are the most representative techniques adopted in the development of hydrogel-based actuators to prepare hydrogels that mimic behaviors from nature, such as self-folding, twisting and bending in the absence of changes of specific environmental stimuli. Therefore, hydrogel actuators have been used in designing micromanipulators,30 sensors,31 optical devices13 and microfluidic devices.32
This review highlights stimuli-responsive hydrogels (stimuli are summarized in Fig. 1), focusing in particularly on shape memory hydrogels (SMHs) and hydrogel actuators (HAs). Main developing trends of SMHs are firstly presented, including toughening SMHs, SMHs with a triple-/multi-shape memory effect and multifunctional SMHs. And then, a brief overview is given of hydrogel actuators with respect to their stimuli, motion-deformations, the technologies and their applications in specific examples.
2. Shape memory hydrogels
2.1 Tough shape memory hydrogels
Because of the weak bonding energy of reversible interactions, SMHs based on only exchangeable bonds usually suffer from poor mechanical properties. However, low strength hydrogels would lose their great potential for use in load-bearing applications. Thus, there is an urgent need to improve the mechanical properties in an appropriate way. Many strategies have been applied to enhance the mechanical properties, including tensile strength, compressive strength and toughness, for contributing to strong hydrogels, which can also be used for SMHs for example constructing particular structures (double network, dual/triple crosslinks in a single network) or doping nanomaterials, etc.It is well known that double network (DN) structures could improve the mechanical properties effectively, in which one network acts as a sacrifice network for energy dissipation and the other serves as an elastic network to keep permanent shapes.33–37 Similarly, SMHs usually have two different crosslinks, either forming a physical network or chemical network, one of which is for fixing temporary shapes and one for maintaining original shapes. Thus, the reversible switches used for the shape memory effect can also play an important part in strengthening the toughness of hydrogels. For example, Weiss et al. prepared a tough hybrid hydrogel having both physical and covalent cross-links, and it can achieve shape memory behavior through the switching of glassy nanodomains at a certain temperature.38 By integrating a physically cross-linked gelatin network and a chemically cross-linked PAAm network with graphene oxide (GO), Tong's group realized NIR-triggered shape memory performance, in which both the gelatin network and the GO bridging take part in dissipating deformation energy.39
According to a similar mechanism of energy dissipation, the construction of dual/triple crosslinks in a single network is another effective approach to render SMHs with good mechanical properties. For instance, in the system designed by Wang et al., strong multiple hydrogen bonds form between poly(vinyl alcohol) (PVA) and tannic acid (TA) molecules, functioning as permanent crosslinks, while weaker hydrogen bonds between PVA chains act as reversible interactions for shape memory and shape recovery. The breaking and the formation of the weaker hydrogen bonds also play the role of sacrificial bonds to endow hydrogels with excellent mechanical properties (Fig. 2).40
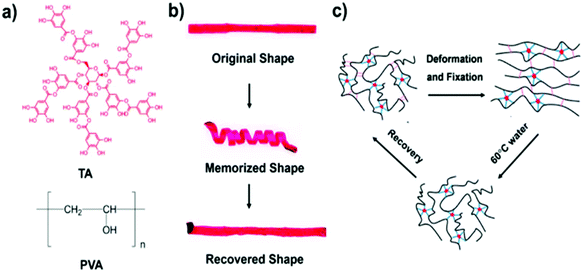 | ||
| Fig. 2 PVA–TA SMHs with excellent mechanical properties (reproduced from ref. 40 with permission of the American Chemical Society). | ||
2.2 Triple-/multi-shape memory hydrogels
Previous SMHs, possessing a dual shape memory effect, can only remember one simple temporary shape in each shape memory cycle. Since the number of temporary shapes that can be fixed will affect the potential applications of SMHs, more and more attention has been paid to the fabrication of triple-/multi-shape memory hydrogels. Nowadays, there are three main ways to endow SMHs with triple-/multi-shape memory effects, which are summarized in the following.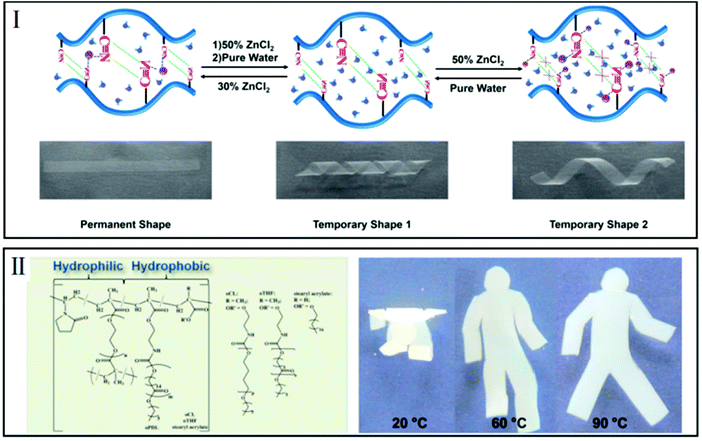 | ||
| Fig. 3 Single-stimuli-induced triple-shape memory effect. (I) Zn2+ triggered triple memory effect by managing the interactions of CN–CN and Zn–CN (reproduced from ref. 41 with permission of John Wiley and Sons); (II) heat-triggered triple shape memory effect at different temperatures because of two types of semicrystalline side chains (reproduced from ref. 42 with permission of the American Chemical Society). | ||
As one of the most commonly used stimuli, heat can be employed to design SMHs with a triple-/multi-shape memory effect due to the different forces generated from different interactions. Hydrogels reported by Lendlein et al. are composed of two different types of hydrophobic crystallizable switching segments, corresponding to two different switching temperatures (Fig. 3-II). During the programming procedure of changing the temperature, two distinct temporary shapes can be memorized and recovered through crystallization and amorphization of hydrophobic side chains.42
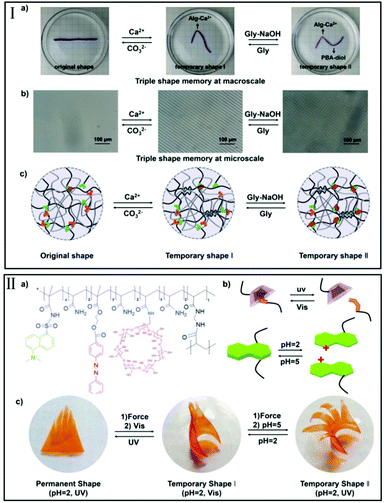 | ||
| Fig. 4 Different stimuli-triggered triple-shape memory effects. (I) Triple-shape memory effects at both the macroscale and microscale have been achieved by programmable introduction of Alg–Ca2+ chelation and PBA–diol ester bonds (reproduced from ref. 43 with permission of the Royal Society of Chemistry); (II) triple-shape memory effect realized by adjusting pH and UV/Vis stimuli (reproduced from ref. 46 with permission of the American Chemical Society). | ||
As shown in Fig. 4-II, a light-, pH-, and thermo-responsive hydrogel with a triple-shape memory effect was prepared by introducing dansyl-aggregations and host–guest interactions on the basis of azobenzene (Azo) with cyclodextrin (β-CD). Since the aggregation of dansyl groups at a high pH value and the supramolecular inclusion complexes of β-CD-Azo under visible light can act as reversible switches, a triple-shape memory effect can be realized in response to light and pH sequentially.46 And, what is more, the reversible aggregation–disaggregation transition of the dansyl group can respond to both pH and temperature, thus the hydrogels showed a triple-shape memory behavior by controlling the programming process.47
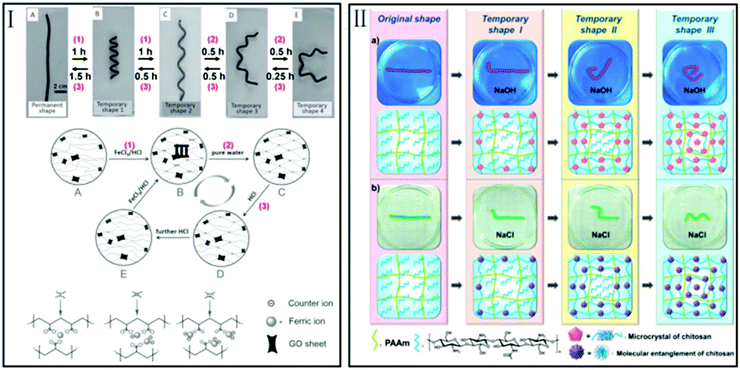 | ||
| Fig. 5 Time-controlled multi-shape memory effect. (I) Adjusting the types of formation between Fe3+ and AAc (mono-, bi-, and tridentate) by changing the immersing time in a FeCl3/HCl or HCl solution for multi-shape memory (reproduced from ref. 48 with permission of John Wiley and Sons); (II) different degrees of formation of microcrystals or molecular entanglement of chitosan for multi-shape memory (reproduced from ref. 49 with permission of MDPI). | ||
2.3 Multifunctional shape memory hydrogels
Since a single function can often not meet all requirements for practical applications, it is quite promising to develop SMHs with multi-functionalities, such as self-healing, self-adhesion, thermoplasticity as well as antibacterial and anti-inflammatory functions.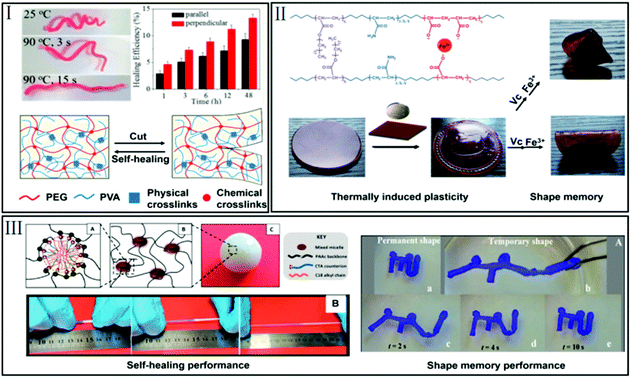 | ||
| Fig. 6 SMHs with other functionalities. (I) SMHs with self-healing behavior (reproduced from ref. 51 with permission of the American Chemical Society); (II) SMHs with thermoplasticity (reproduced from ref. 52 with permission of the Royal Society of Chemistry); (III) SMHs with self-healing and high mechanical strength (reproduced from ref. 53 with permission of the American Chemical Society). | ||
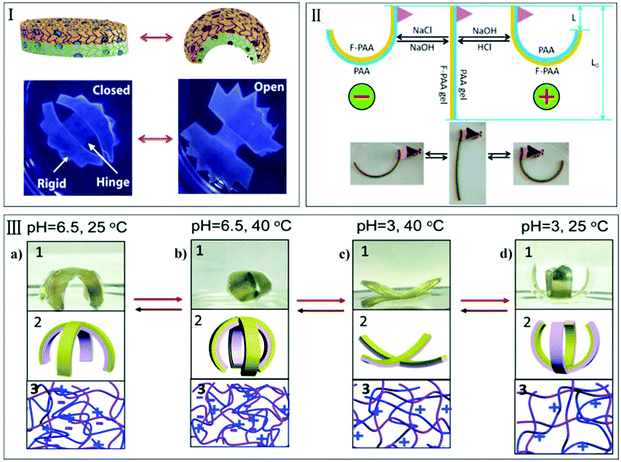
In addition, another anisotropic hydrogel with integrated self-deformation and controllable shape memory effect has also been reported by Chen's group. Through constructing an anisotropic PAAc–PAAm structure, the obtained hydrogel deformed into certain shapes in response to pH stimulus, which can be fixed by the coordination between carboxylic groups and Fe3+. Moreover, the shape memory ratio and shape recovery ratio could be adjusted with the concentration of the corresponding ions, such as Fe3+ and H+.40
3. Stimuli-responsive hydrogel actuators
3.1 Design principles and strategies for fabricating a hydrogel-based actuator
The main principle of movement in nature tissue is based on the discrepant part in nonuniform swelling. In order to create a self-folding deformation, a generated bending in a hydrogel sheet resulting from differential stress either along its thickness or its lateral dimensions is necessary.57 Up to now, several strategies have been developed to fabricate smart hydrogels with inhomogeneous structures. One approach is to fabricate discrepant distributions in the structure of responsive hydrogels (Fig. 7), such as (i) a hydrogel with inhomogeneous cross-linked dots, which generates deformation when asymmetrical swelling occurs due to highly crosslinked dots within a low crosslinked matrix allowing cross-linking to be adjusted by irradiation dose58 (Fig. 7-I), (ii) “bistrip” hydrogels with variations in the degree of crosslinking and hence possessing varying degrees of swelling making the hydrogel to shrink, unroll and recover a flat shape when the temperature of the aqueous medium changes59 (Fig. 7-II), (iii) imprinting Cu2+ ion complexes locally with anionic hydrogels to fabricate stiffer ionoprinted regions, which guide the asymmetric shrinking and reshaping of the gel structure60 (Fig. 7-III), or (iv) heterogeneous porous structures by using a one-step evaporation process to create a laminated layer/porous layer heterogeneous structure in a vertical direction and pattern the heterogeneous structure in a lateral direction to form tunable, fast, and robust hydrogel actuators61 (Fig. 7-IV). Typically, variation in irradiation time endows the hydrogel regions with different crosslinking densities and hence the resulting hydrogel areas show different swelling/deswelling properties, which further result in the generation of in-plane stress. As a result, shape deformations occur in the hydrogel matrix. This means that the shape deformation of the hydrogels can be programmed, e.g., by using variations of photolithographic patterns to generate areas with different crosslinking degrees or to change the structure of surface features to yield asymmetric shrinking and reshaping.62,63 Another straightforward strategy is to create a bilayer hydrogel with discrepant swelling layers in the longitudinal axis, which generates bending stress due to the differences in swelling degrees.64–67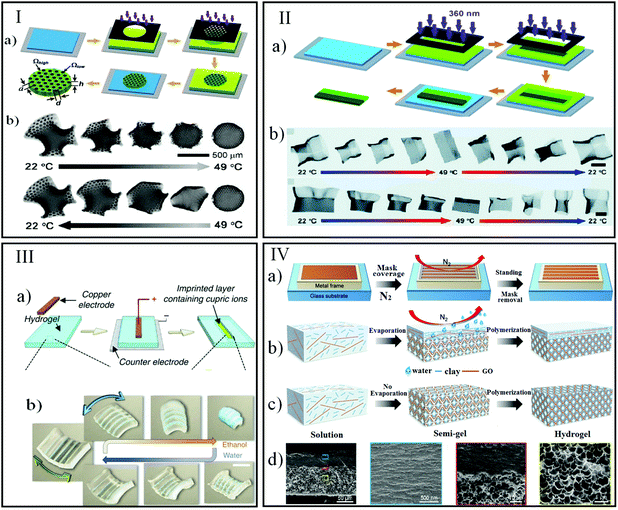 | ||
| Fig. 7 Fabrication and swelling-induced deformation of inhomogeneous hydrogels: (I) highly crosslinked dots within a low crosslinked halftone gel matrix. Reproduced with permission from ref. 58. Copyright 2012, American Association for the Advancement of Science; (II) hydrogel with variations in the degree of crosslinking. Reproduced with permission from ref. 59. Copyright 2012, Royal Society of Chemistry; (III) patterned stiffer ionoprinted regions on anionic hydrogels causing asymmetric shrinking and reshaping of the gel structure in EtOH (ethanol) and water. Reproduced with permission from ref. 60. Copyright 2013, Nature Communications; (IV) heterogeneous porous structures by using a one-step evaporation process to create a laminated layer/porous layer heterogeneous structure in a vertical direction. Reproduced with permission from ref. 61. Copyright 2017, American Chemical Society. | ||
Here, some examples of bilayer hydrogels used for actuators are listed. Noy Bassik et al.64 fabricated hydrogel bilayers, derived from N-isopropyl-acrylamide (NIPAM), acrylic acid (AA), and poly(ethylene oxide)diacrylate (PEODA), which can fold and unfold in response to changing aqueous solutions having different pH values and ionic strengths (Fig. 8-I). These actuators are composed of two layers with different swelling behaviors and were prepared by combining spin coating and photolithographic patterning techniques. Of these two layers, the pH-responsive layer swells in solution with a pH above the pKa of the hydrogel due to the ionization and subsequent dissociation of the acid group in the hydrogel, whereas the passive layer has no response. As a result, an osmotic pressure generated between the surface and inside the hydrogel causes the folding behavior due to the difference in mobile ion concentration as governed by the Donnan equilibrium. Zhao et al.65 prepared bilayer- and multilayer-hydrogel actuators using a PAA gel reinforced by further cross-linking with Fe3+ ions (Fig. 8-II). These hydrogel actuators showed a rapid response and a precise control of the direction of action in the media having different pH values and ionic strengths. Li et al.66 fabricated temperature- and pH-responsive semi-interpenetrating network (semi-IPN) hydrogel-based bilayer actuators by generating a PNIPAM based hydrogel in the presence of PDADMAC on a layer of gold-coated polydimethylsiloxane (PDMS) (Fig. 8-III). These bilayers exhibit a revisable and repeatable thermoresponsive and pH-responsive bending and unbending behavior and can be used as soft grippers.
 | ||
| Fig. 8 Bilayer hydrogel actuators. (I) Actuator with hinges folding in changing pH/IS. A VF-shaped actuator constructed from rigid SU-8 segments with a NIPAm–AAc/PEODA bilayer hinge (reproduced from ref. 64 with permission. Copyright 2010, Elsevier Ltd); (II) reversible actuation of the Janus bilayer hydrogel in different solutions (reprinted with permission from ref. 65. Copyright 2017, American Chemical Society); (III) a temperature- and pH-responsive semi-interpenetrating network (semi-IPN) hydrogel-based bilayer actuator (reprinted with permission from ref. 66. Copyright 2017, Royal Society of Chemistry). | ||
3.2 Stimulation of “smart” hydrogels
“Smart” hydrogels with controllable volume/shape changes in response to external physical or chemical/biochemical stimuli have become promising stars in developing mimetic actuators for controllably triggered manipulation.68–73 In general, physical stimuli, including temperature,74 electrical signals,75 magnetic fields,17 or mechanical stress,76 alter molecular interactions at critical onset points. However, chemical stimuli, such as pH,77 ionic factors,78 and chemical agents, will change the interactions between polymer chains or between polymer chains and solvent at the molecular level. Recently, biochemical stimuli have been considered as another category and involves responses to antigens,79 enzymes,80 ligands,81 and other biochemical agents.82–84 In order to explore and mimic the more complex stimulation systems from nature, more researchers have developed novel hydrogel systems in which two or more stimuli-responsive mechanisms are combined, the so-called dual-responsive and multi-responsive hydrogel systems.85–88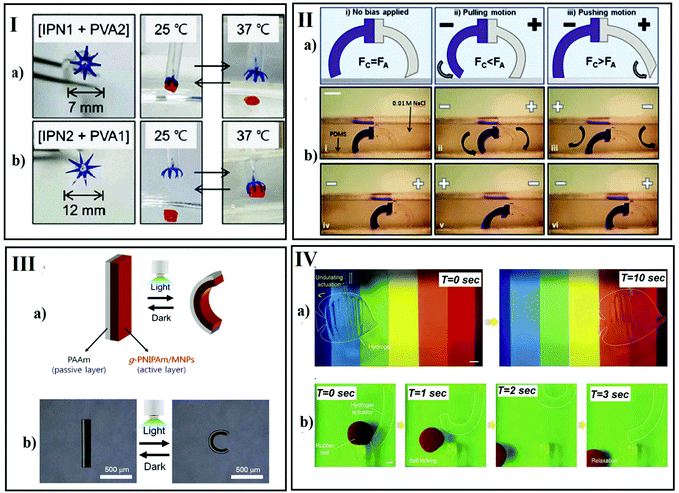 | ||
| Fig. 9 Hydrogel actuators induced by physical stimuli. (I) Temperature-responsive starfish-shaped gripper. Reprinted with permission from ref. 103. Copyright 2018, Polymer Society of Korea and Springer; (II) light-responsive bidirectional and circular bending actuator. Reprinted with permission from ref. 105. Copyright 2016, Nature publication; (III) hydrogel walker moving in the direction of the applied electric field. Reprinted with permission from ref. 114. Copyright 2014, Royal Society of Chemistry; (IV) hydraulic hydrogel actuators. Reprinted with permission from ref. 121. Copyright 2017, Nature publication. | ||
Using an electrical signal as an environmental stimulus to induce responses in hydrogels has received particular attention due to the reliable control of signal strength and direction, remote operation and a precise “on/off” triggering switch. Typical hydrogels that are sensitive to an electrical signal are polyelectrolyte networks, such as polysaccharide and poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS). Under electric fields, osmotic pressure gradients are produced due to the asymmetrical distribution of mobile ions in polyelectrolyte networks (electrolyte solution), which induce the shrinking or swelling of the polyelectrolyte hydrogel. In 1965, Hamlen et al.104 observed the expansion and contraction of a poly(vinyl alcohol-co-acrylic acid) polyelectrolyte gel induced by an electric field. Since then, an increasing number of electrically-controlled artificial muscles, robots and other smart devices were fabricated using polyelectrolyte polymers based on the electro-induced volume/shape changes.105–107 For example, Yang et al.105 developed soft hydrogel walkers consisting of polyanionic poly(2-acrylamide-2-methylpropanesulfonic acid-co-acrylamide) (P(AMPS-co-AAm)) exhibiting an arc looper-like shape with two “legs” for walking in an electrolyte solution. Morales et al.106 fabricated gel actuators with two legs composed of gels that can walk unidirectionally in diluted salt solutions without the need for ratchet surfaces to the electric field (Fig. 9-II).
Light as another promising stimulus was investigated in photo-driven hydrogel actuators benefiting from their numerous advantages such as fast response, remote operation, easy to downsize, controllable intensity, no necessary physical contact with the materials and high efficiency. Photo-responsive hydrogels can be classified into UV-sensitive and visible-light-sensitive hydrogels. Irradiation from a unique light source, the changes in polarity, free volume, and hydrophilicity/hydrophobicity of photo-responsive hydrogel actuators endow them with the corresponding possibility of motion. As reported, typical photo-driven hydrogels were synthesized by introducing photo-sensitive chromophores, such as azobenzene (due to their cis–trans photo-isomerization)108 or spiropyran (ring opening and closing photo-isomerization)109,110 into the side or main chains of polymers. Additionally, photo-thermal conversion materials dispersed in thermally responsive hydrogel matrices have been demonstrated to be sufficient for transferring the energy from visible or near-infrared light to thermal energy, further resulting in a volume phase transition of hydrogels. In this case, the main trigger is thermal heat generation by absorption of light in the hydrogel matrix, which triggers volume shrinkage of the temperature-responsive hydrogel. Most research on these photo-thermal materials focuses on carbon-based materials, such as graphene oxide (GO)111 and metallic nanoparticles such as iron oxide112 or gold.113 For example, Lee et al.114 fabricated a fast responding photo-actuator by combining a comb-type PNIPAM hydrogel matrix with magnetite nanoparticles (Fig. 9-III).
Similar to light and an electrical signal, an external magnetic field can also be used to remotely control a magnetically-active hydrogel rapidly. Typically, a magnetically-active hydrogel consists of composites of hydrogel networks filled with magnetic nanoparticles, resulting in direct coupling between the magnetic and the mechanical properties of the magnetically-active polymeric gel, such as distributed metal particles, iron(III) oxide particles115 or ferromagnetic particles116,117 attached to the flexible polymer network via hydrogen bonding, van-der-Waals forces or, coordination bonds. The rapid and controllable shape changes of these gels would be expected to mimic muscular contraction. Combined with biodegradable and biocompatible polymers, such as poly(D,L-lactide), poly(ε-caprolactone), poly(ethylene glycol) and their copolymer and grafting polymers, these magnetically-active hydrogels have originally been proposed for biomedical applications.118–120
Besides the above-mentioned stimuli, pressure is also of interest as an induced trigger for smart hydrogels. Thus, for example, Yuk et al.121 have, inspired by leptocephali, developed hydraulic hydrogel actuators, which can imitate the camouflage capabilities optically and sonically. These hydrogel actuators are also able to kick a ball and catch a live fish owing to the anti-fatigue properties of the hydrogel under moderate stresses (Fig. 9-IV).
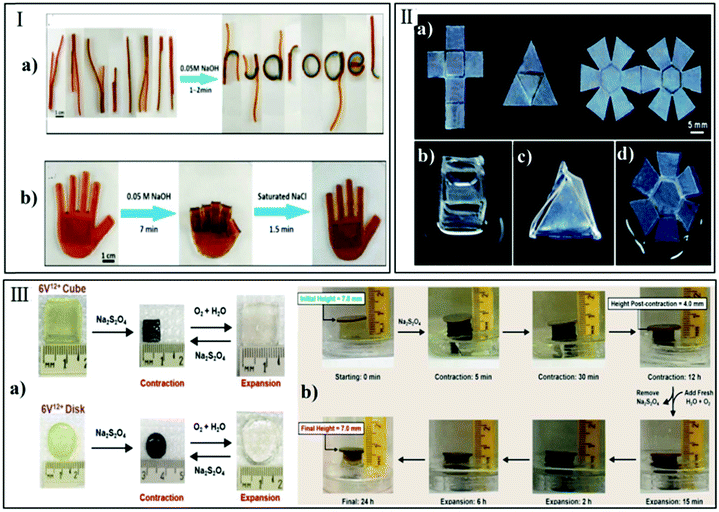 | ||
| Fig. 10 Hydrogel actuators induced by chemical stimuli. (I) Gel strips assembled by a pH-responsive hydrogel actuator to represent the word hydrogel and the grasp-open action of the hand-shaped actuator. Reprinted with permission from ref. 65. Copyright 2017, American Chemical Society; (II) hexane-responsive hydrogels folded themselves from (a) programmed 2D structures to transformed 3D objects in hexane solvent: (b) cube, (c) pyramid, and (d) phlat ball. Reprinted with permission from ref. 125. Copyright 2011, Royal Society of Chemistry; (III) demonstration of an artificial molecular muscle at work in versene solution and H2O. Reprinted with permission from ref. 128. Copyright 2017, American Chemical Society. | ||
Changing the composition of the solvent in hydrogels, such as a hydrophilic or a hydrophobic chemical solvent, also induced bending and twisting behavior of the hydrogel. For instance, Jeong et al.125 fabricated reversible and color-tunable photonic actuators responding to both hydrophilic and hydrophobic chemical environments (acetic acid and hexane) through a symmetry breaking process and a change in the lattice constant of the photonic crystal structure. In Fig. 10-II, the 2D programmed structure transformed to a 3D cubic object in hexane.
Redox-responsive hydrogels have always been regarded as one of the most promising biomaterial composites for biomedical applications, and have frequently been employed in areas, such as tissue engineering and drug delivery.126,127 Particularly poly(amido amine)-based redox-responsive hydrogels have, due to their property of being biocompatible and highly versatile, gained significant attention. Greene et al.128 introduced a redox-responsive mechanism of actuation in hydrogels by describing a systematic investigation into the radical-based self-assembly of a series of unimolecular viologen-based oligomeric links. Moreover, an artificial molecular muscle at work was demonstrated (Fig. 10-III).
Besides the biochemical stimuli mentioned above, antigen enzyme-triggered hydrogels used in biomedical areas have been substantially further developed.141–143 For example, Athas et al.141 designed “hybrid” hydrogels with a Venus flytrap shape, derived from three individual gels with distinct properties, which can transform from an open to a closed state upon exposure to enzyme, i.e., collagenase or lysate of murine fibroblast cells.
3.3 Fabrication technologies adopted in the development of hydrogel-based actuators
Depending on the application and design shape of hydrogel actuators, their dimensions range from nanometers to centimeters. As a result, different fabrication technologies, such as molding, micromolding, dip-coating, lithography, 3D printing, ionoprinting, capillary origami and ion inkjet printing, were utilized in the development of hydrogel-based actuators and are summarized in the following.150Photolithography is composed of operating a mask with the desired geometric patterns and subsequently transferring the pattern to light-sensitive materials via irradiation with ultraviolet light. Based on this strategy, Kumar et al.156 formed self-rolled polymer bilayer microtubes. A polymer bilayer was first created by consecutive deposition of poly(4-vinylpyridine) (P4VP) from chloroform and poly(4-bromostyrene) (BrPS) dissolved in toluene on a silicon wafer, by dip-coating. Then, photolithography was used for patterning the bilayer with a UV lamp having a 2.5 W output at 254 nm to irradiate the P4VP/BrPS bilayer. Here, TEM copper grids were used as a photomask, and UV irradiation caused crosslinking between P4VP and BrPS in the irradiated area. The geometric patterns were developed by washing the uncured polymer with a solvent. Such methods rely primarily on photopolymerization of liquid precursors, hence they are only appropriate for photocrosslinkable hydrogels. Moreover, for the fabrication of micro-dimensions in such processes (e.g., microfluidics, microchannels), complicated and expensive external devices or platforms in a special cleaning room are required (Fig. 11).
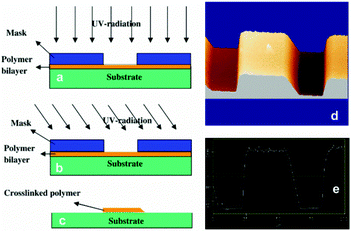 | ||
| Fig. 11 The photolithography process and schematic way of formation of asymmetric pattern. Reprinted & reproduced with permission from ref. 156. Copyright (2008), Elsevier. | ||
Soft lithography, which employs elastomeric stamps fabricated from patterned silicon wafers to print or mold materials, is commonly used in 3D microstructure fabrication.157 Besides that, the combination of two lithographic methodologies has been used in fabricating nano- and microstructures to control the geometric size and shape of hydrogels. Thus, for example, a method for preparing PEG or PEGDA hydrogel structures using electron beam lithography and ultraviolet optical lithography as nano- and micro-fabrication techniques has been reported.158 Here, E-beam lithography operates with a very high resolution for small structures with a low rate of production. Conversely, UV lithography yields a much higher rate of production, but the resolution is lower. However, with these above-mentioned methods it is difficult to prepare large-sized masks or templates with elaborate patterns, especially those with well-defined, programmable variation in crosslinking densities.62
 | ||
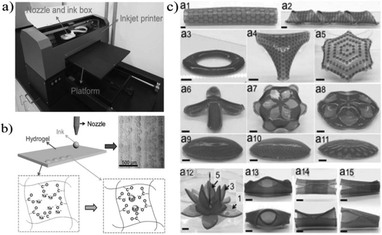
| Fig. 12 3D printing technologies: (I) 3D printed hydrogel beam actuator (reprinted & reproduced with permission from ref. 161. Copyright 2017, Elsevier B.V.); (II) computer-aided design model of hydrogel valve and its swollen and shrunken behaviors in water at 20 °C and 60 °C, respectively (reprinted with permission from ref. 163. Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA). | ||
 | ||
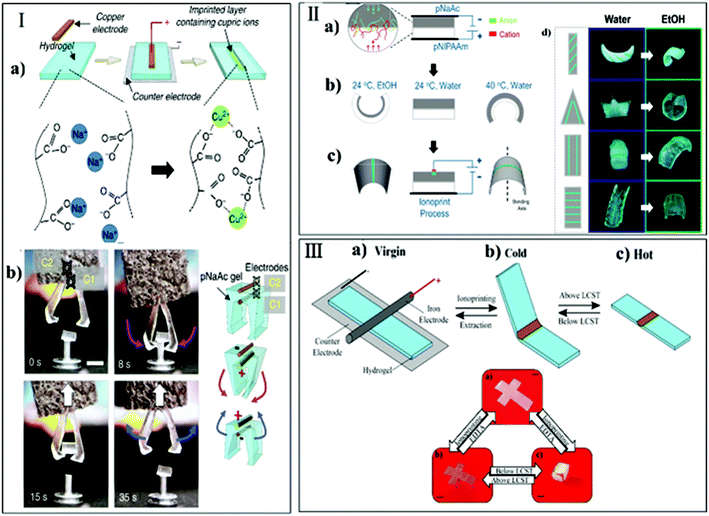
| Fig. 13 Ion inkjet printing technique: (a) photograph of flatbed inkjet printer, (b) schematic of the inkjet printing process and image of printed dots on the hydrogel surface as well as cross-linking between ferric ions and anionic carboxyl groups of PNaAAc; (c) complex 3D shapes deformed from 2D patterned hydrogel sheets, including 3D shapes deformed from hydrogels with patterns printed on only one surface (reprinted with permission from ref. 146. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA). | ||
 | ||
| Fig. 14 The ionoprinting technique. (I) Patterned Cu anode with anionic hydrogels to fabricate an ionoprinted hydrogel cylinder. Reprinted with permission from ref. 60. Copyright 2013, Nature Communications; (II) a hydrogel bilayer bending axis orthogonal to the ionoprint direction and bending axis between water and EtOH. Reprinted with permission from ref. 63. Copyright 2016, MDPI; (III) an ionoprinted hydrogel folded and unfolded, oscillating between below LCST and above LCST, respectively (reprinted with permission from ref. 165. Copyright 2017, Elsevier B.V.). | ||
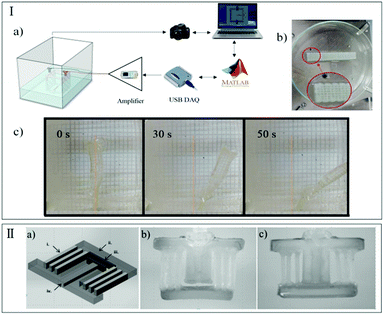
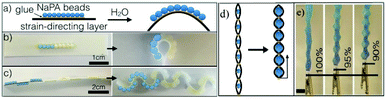 | ||
| Fig. 15 Schematic of the HARICOT. (a) Illustrated schematic. (b and c) Actuators with NaPA beads attached to the surface and the opposite parts of the paper layer before and after the swelling. (d) Schematics of the working principle of the muscle-like actuator; (e) example of a muscle, before and after the swelling (reprinted with permission from ref. 169. Copyright 2017, Elsevier Ltd). | ||
3.4 Application
Stimuli-responsive hydrogels have been used in designing micromanipulators, sensors, and optical devices with tunable focal length, in control of liquid flow in microfluidic devices, etc. For example, Kim et al.167 developed a hydrogel actuator that walks in one direction by using alternating cycles of heating and cooling (Fig. 16-I), unlike the unidirectionally proceeding soft actuator with an external physical bias, such as ratchet-patterned bases or directional stimuli. Here a hydrogel actuator with an L-shaped symmetrical bipedal walker was designed to adopt a layered structure with cofacially oriented unilamellar electrolyte titanate(IV) nanosheets, with the tilt direction of the titanate(IV) nanosheets identifying the forefoot and the backfoot. Upon heating from 25 °C to 45 °C, the backfoot of this object elongates and kicks the base, causing the object to walk forward via an internal mechanism for anisotropic energy conversion, meanwhile, its center of mass shifts from its bend point towards the front side. When cooling, the object shortens its elongated backfoot and is drawn backward. However, interestingly, the center of mass shift can potentially suppress this backward motion. Inspired by living bio-actuators such as a stalk in Vorticella, Yoshida et al.168 applied a spring-shaped structure to fabricate a hydrogel actuator to magnify its deformation degree (Fig. 16-II). The large compression/expansion motion of such a micro-spring can be controlled by changing the pattern of a stimuli-responsive hydrogel component. These stimuli-responsive hydrogel microsprings have immense potential to be applied in various micro-engineering products, including soft actuators, chemical sensors, and medical applications. A living wearable patch that detects chemicals on the skin was fabricated by a bilayer hybrid structure of the hydrogel and elastomer, which can conformally attach to the human skin.169 Such a hydrogel–elastomer hybrid has four isolated chambers hosting different bacterial strains that can sense their cognate inducers (i.e., N-acyl homoserine lactone, isopropyl β-D-1-thiogalactopyranoside, and rhamnose) visually. In Fig. 16-III, the hand with an inducer showed a fluorescent glow. Hence, these kinds of functional living devices can be potentially utilized in chemical detection, scientific research and translational medicine in the future. Using thermo-responsive hydrogel composites with graphene oxide nanosheets, Wang et al.170 created light-controlled nanocomposite hydrogel actuators that exhibit diverse mechanical motions by controlling their shape and surface patterns. By modulating laser positioning, timing, and movement, the patterned hand-shaped matrix created joint-like flexing motions upon sequential irradiation (Fig. 16-IV). Surprisingly, Yuk et al.121 developed an agile and camouflaged hydrogel-based actuator to imitate the capabilities of leptocephali, which are high-speed, high-force, and being optically and sonically camouflaged in water. What is even more interesting is that these agile and transparent hydrogel actuators can perform extraordinary functions, like robots, including a hydrogel robotic fish that can achieve forward fishlike locomotion (swimming), an actuator that can kick off a ball, and a camouflaged hydrogel gripper that can even catch and release a live goldfish (Fig. 16-V). Inspired by the focusing strategy of a human lens, a bioinspired flying saucer-like smart lens with a tunable-focus adjusted by the swelling/deswelling behaviors of a bilayer hydrogel was fabricated (Fig. 16-VI).151 Mimicking the human lens with an asymmetric biconvex structure connected to a ciliary muscle, such a bioinspired lens device consisted of two “caps”. These caps are shrunk under a high pH value, leading to flattening, similar to the human lens which relaxes when the person it belongs to has a rest and makes the crystalline lens appear ‘flattened’, whereas, the caps are swollen under a low pH value, leading to the rounded shape. These optical lenses with a tunable focus via adjusting the flattened- and rounded-state are required for potential application in many fields, such as consumer electronics, medical diagnostics, and optical communications. A microfluidic system like a micro-valve to control the liquid flow is one of the potential applications of a micro-hydrogel actuator. Another example is to use the microfluidic channel to sort and guide droplets generated by using a cascading droplet generation channel to demonstrate the visual operation of the electroactive hydrogel actuator (Fig. 16-VII).10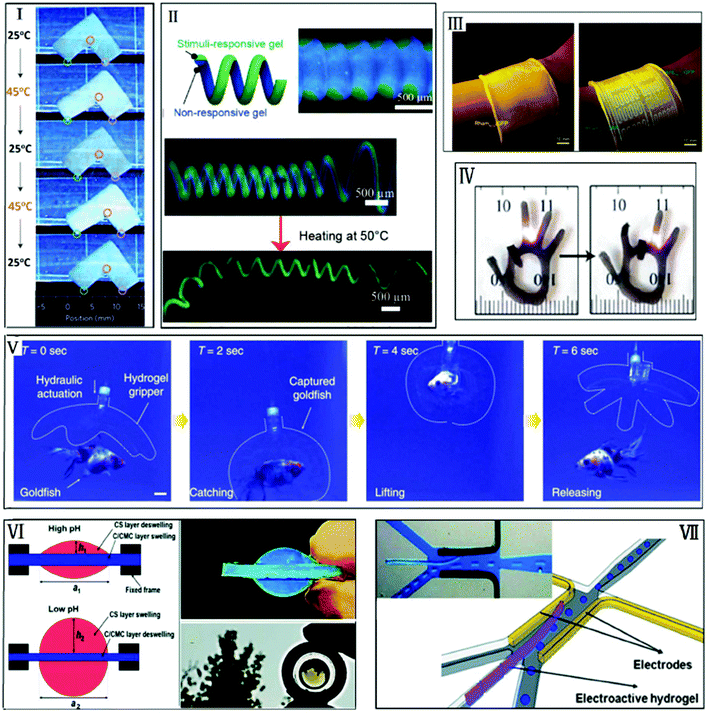 | ||
| Fig. 16 Examples of hydrogel actuators used in several applications: (I) an L-shaped hydrogel walker fabricated from a PNIPA hydrogel with oriented unilamellar electrolyte titanate nanosheets which can act as a unidirectionally proceeding actuator. Reprinted from ref. 167 with permission, Copyright 2015, Nature publication; (II) a microspring based on an alginate and poly(N-isopropylacrylamide-co-acrylic acid) hydrogel. Reprinted with permission from ref. 168. Copyright 2017, Nature publication; (III) a hydrogel–elastomer hybrid with four isolated chambers hosting different bacterial strains to sense their cognate inducers visually. Reprinted with permission from ref. 169. Copyright 2017, United States National Academy of Sciences; (IV) joint-like flexing motions of a light-controlled nanocomposite hydrogel hand, reprinted with permission from ref. 170. Copyright 2013, American Chemical Society; (V) agile and transparent hydrogel gripper for catching and releasing a live goldfish. Reprinted with permission from ref. 121. Copyright 2017, Nature publication; (VI) a bioinspired lens based on a bilayer hydrogel. Reprinted with permission from ref. 151, Copyright 2017, The Royal Society of Chemistry; (VII) a microfluidic channel based on an electroactive hydrogel to sort and guide droplets. Reprinted with permission from ref. 10, Copyright 2010, Royal Society of Chemistry. | ||
4 Summary and outlook
Nature inspired us with a variety of ideas to develop novel structures and to mimic natural properties, such as pinecone seeds that fall down because the hydrated pinecone has dried up. Based on the phenomena of reversible “on–off” switches, scientists learned the mechanism and principles of various motions in nature and further transferred the knowledge from nature to synthetic materials for designing hydrogel-based smart actuators. Then, attempts to utilize these artificial hydrogel actuators in different possible applications, such as bioapplications, have been developed. In this review, we presented two kinds of remarkable “smart” hydrogels inspired by nature, i.e., shape memory hydrogels (SMHs) and stimuli-responsive hydrogels (actuators) (SRHs). We also presented different types of SMHs and discussed the effect individual factors have on shape memory. Additionally, the stimuli-responsive hydrogels used to fabricate actuators have been illustrated and exemplified depending on the types of environmental stimuli, such as physical, chemical and biochemical stimuli. Furthermore, several strategies for manufacturing smart hydrogels with inhomogeneous structures and fabrication technologies adopted in hydrogel-based actuators have been discussed and summarized. Yet there is still a long way to go in producing complex deformations from 2D to 3D, just like general and collaborative multi-step actuation in natural life, partly because chemical structures sensitive to multi-stimuli should be combined in a hydrogel matrix to achieve multi-step actuation imitating natural life. Furthermore, intrinsic interest on new and different chemical approaches and their external stimulation should be investigated more. In addition, the sequence control and the time-consuming process of hydrogels with a complex deformation are still challenging to achieve with the current fabrication techniques. Hence, trends of “smart” hydrogels are to develop the formation of multi-stimulative hydrogels with complex but programmed self-folding, twisting and bending behaviors by designing inhomogeneous hydrogels through the creation of improved fabrication technologies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. S. gratefully acknowledges the China Scholarship Council (CSC grant: 201406240131) for partial financial support of this work.References
- R. Fernandes and D. H. Gracias, Adv. Drug Delivery Rev., 2012, 64, 1579–1589 CrossRef CAS PubMed.
- M. A. Ward and T. K. Georgiou, Polymer, 2011, 3, 1215–1242 CAS.
- T. Kumar Giri, D. Thakur, Ajazuddin, H. Badwaik and D. Krishna Tripathi, Curr. Drug Delivery, 2012, 9, 539–555 CrossRef.
- I. Strehin, Z. Nahas, K. Arora, T. Nguyen and J. Elisseeff, Biomaterials, 2010, 31, 2788–2797 CrossRef CAS PubMed.
- P. N. Charron, S. L. Fenn, A. Poniz and R. A. Oldinski, J. Mech. Behav. Biomed. Mater., 2016, 59, 314–321 CrossRef PubMed.
- N. T. Phuong, V. A. Ho, D. H. Nguyen, N. C. Khoa, T. N. Quyen, Y. Lee and K. D. Park, J. Bioact. Compat. Polym., 2015, 30, 412–423 CrossRef.
- R. Jin and P. J. Dijkstra, Biomed. Appl. Hydrogels Handb., 2010, 101, 203–225 Search PubMed.
- I. Vasiev, A. I. M. Greer, A. Z. Khokhar, J. Stormonth-Darling, K. E. Tanner and N. Gadegaard, Microelectron. Eng., 2013, 108, 76–81 CrossRef CAS.
- S. Zakharchenko, N. Puretskiy, G. Stoychev, M. Stamm and L. Ionov, Soft Matter, 2010, 6, 2633–2636 RSC.
- G. H. Kwon, Y. Y. Choi, J. Y. Park, D. H. Woo, K. B. Lee, J. H. Kim and S. H. Lee, Lab Chip, 2010, 10, 1604–1610 RSC.
- S. Maeda, Y. Hara, R. Yoshida and S. Hashimoto, Int. J. Mol. Sci., 2010, 11, 52–66 CrossRef CAS PubMed.
- C. Ma, W. Lu, X. Yang, J. He, X. Le, L. Wang, J. Zhang, M. J. Serpe, Y. Huang and T. Chen, Adv. Funct. Mater., 2018, 28, 1704568 CrossRef.
- A. Mateescu, Y. Wang, J. Dostalek and U. Jonas, Membranes, 2012, 2, 40–49 CrossRef CAS PubMed.
- M. Hasnat Kabir, T. Hazama, Y. Watanabe, J. Gong, K. Murase, T. Sunada and H. Furukawa, J. Taiwan Inst. Chem. Eng., 2014, 45, 3134–3138 CrossRef CAS.
- H. Ko and A. Javey, Acc. Chem. Res., 2017, 50, 691–702 CrossRef CAS PubMed.
- M. P. M. Dicker, A. B. Baker, R. J. Iredale, S. Naficy, I. P. Bond, C. F. J. Faul, J. M. Rossiter, G. M. Spinks and P. M. Weaver, Sci. Rep., 2017, 7, 9197 CrossRef PubMed.
- T. Shen, M. G. Font, S. Jung, M. L. Gabriel, M. P. Stoykovich and F. J. Vernerey, Sci. Rep., 2017, 7, 1–10 CrossRef PubMed.
- B. Q. Y. Chan, Z. W. K. Low, S. J. W. Heng, S. Y. Chan, C. Owh and X. J. Loh, ACS Appl. Mater. Interfaces, 2016, 8, 10070–10087 CrossRef CAS PubMed.
- W. Lu, X. Le, J. Zhang, Y. Huang and T. Chen, Chem. Soc. Rev., 2017, 46, 1284–1294 RSC.
- C. Löwenberg, M. Balk, C. Wischke, M. Behl and A. Lendlein, Acc. Chem. Res., 2017, 50, 723–732 CrossRef PubMed.
- W. Wang, Y. Zhang and W. Liu, Prog. Polym. Sci., 2017, 71, 1–25 CrossRef.
- R. Luo, J. Wu, N. D. Dinh and C. H. Chen, Adv. Funct. Mater., 2015, 25, 7272–7279 CrossRef CAS.
- Y. Zhang, J. Liao, T. Wang, W. Sun and Z. Tong, Adv. Funct. Mater., 2018, 28, 1–9 Search PubMed.
- J. You, S. Xie, J. Cao, H. Ge, M. Xu, L. Zhang and J. Zhou, Macromolecules, 2016, 49, 1049–1059 CrossRef CAS.
- K. Peng, H. Yu, H. Yang, X. Hao, A. Yasin and X. Zhang, Soft Matter, 2017, 13, 2135–2140 RSC.
- T. Tanaka, E. Sato, Y. Hirokawa, S. Hirotsu and J. Peetermans, Phys. Rev. Lett., 1985, 55, 2455–2458 CrossRef CAS PubMed.
- S. Ma, B. Yu, X. Pei and F. Zhou, Polymer, 2016, 98, 516–535 CrossRef CAS.
- C. Yao, Z. Liu, C. Yang, W. Wang, X. J. Ju, R. Xie and L. Y. Chu, ACS Appl. Mater. Interfaces, 2016, 8, 21721–21730 CrossRef CAS PubMed.
- Z. J. Wang, C. N. Zhu, W. Hong, Z. Liang Wu and Q. Zheng, Sci. Adv., 2017, 3, e1700348 CrossRef PubMed.
- H. Wang, Q. Shi, T. Yue, M. Nakajima, M. Takeuchi, Q. Huang and T. Fukuda, Int. J. Adv. Robot. Syst., 2014, 11, 115–127 CrossRef.
- A. Richter, G. Paschew, S. Klatt, J. Lienig, K. F. Arndt and H. J. P. Adler, Sensors, 2008, 8, 561–581 CrossRef CAS PubMed.
- S. Zhao, Y. Chen, B. P. Partlow, A. S. Golding, P. Tseng, J. Coburn, M. B. Applegate, J. E. Moreau, F. G. Omenetto and D. L. Kaplan, Biomaterials, 2016, 93, 60–70 CrossRef CAS PubMed.
- T. Matsuda, T. Nakajima, Y. Fukuda, W. Hong, T. Sakai, T. Kurokawa, U. Il Chung and J. P. Gong, Macromolecules, 2016, 49, 1865–1872 CrossRef CAS.
- Y. Zhao, T. Nakajima, J. J. Yang, T. Kurokawa, J. Liu, J. Lu, S. Mizumoto, K. Sugahara, N. Kitamura, K. Yasuda, A. U. D. Daniels and J. P. Gong, Adv. Mater., 2014, 26, 436–442 CrossRef CAS PubMed.
- T. C. Suekama, J. Hu, T. Kurokawa, J. P. Gong and S. H. Gehrke, ACS Macro Lett., 2013, 2, 137–140 CrossRef CAS.
- Y. Bu, H. Shen, F. Yang, Y. Yang, X. Wang and D. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 2205–2212 CrossRef CAS PubMed.
- T. Nakajima, H. Sato, Y. Zhao, S. Kawahara, T. Kurokawa, K. Sugahara and J. P. Gong, Adv. Funct. Mater., 2012, 22, 4426–4432 CrossRef CAS.
- J. Hao and R. A. Weiss, ACS Macro Lett., 2013, 2, 86–89 CrossRef CAS.
- J. Huang, L. Zhao, T. Wang, W. Sun and Z. Tong, ACS Appl. Mater. Interfaces, 2016, 8, 12384–12392 CrossRef CAS PubMed.
- X. X. Le, Y. C. Zhang, W. Lu, L. Wang, J. Zheng, I. Ali, J. W. Zhang, Y. J. Huang, M. J. Serpe, X. T. Yang, X. D. Fan and T. Chen, Macromol. Rapid Commun., 2018, 39, 1–6 CrossRef PubMed.
- Y. Han, T. Bai, Y. Liu, X. Zhai and W. Liu, Macromol. Rapid Commun., 2012, 33, 225–231 CrossRef CAS PubMed.
- U. Nöchel, M. Behl, M. Balk and A. Lendlein, ACS Appl. Mater. Interfaces, 2016, 8, 28068–28076 CrossRef PubMed.
- X. Le, W. Lu, J. Zheng, D. Tong, N. Zhao, C. Ma, H. Xiao, J. Zhang, Y. Huang and T. Chen, Chem. Sci., 2016, 7, 6715–6720 RSC.
- H. Xiao, W. Lu, X. Le, C. Ma, Z. Li, J. Zheng, J. Zhang, Y. Huang and T. Chen, Chem. Commun., 2016, 52, 13292–13295 RSC.
- X. Le, W. Lu, H. Xiao, L. Wang, C. Ma, J. Zhang, Y. Huang and T. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 9038–9044 CrossRef CAS PubMed.
- Y.-Y. Xiao, X.-L. Gong, Y. Kang, Z.-C. Jiang, S. Zhang and B.-J. Li, Chem. Commun., 2016, 52, 10609–10612 RSC.
- X.-L. Gong, Y.-Y. Xiao, M. Pan, Y. Kang, B.-J. Li and S. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 27432–27437 CrossRef CAS PubMed.
- L. Zhao, J. Huang, T. Wang, W. Sun and Z. Tong, Macromol. Mater. Eng., 2017, 302, 1600359 CrossRef.
- H. Xiao, C. Ma, X. Le, L. Wang, W. Lu, P. Theato, T. Hu, J. Zhang and T. Chen, Polymer, 2017, 9, 138–148 Search PubMed.
- H. Meng, P. Xiao, J. Gu, X. Wen, J. Xu, C. Zhao, J. Zhang and T. Chen, Chem. Commun., 2014, 50, 12277–12280 RSC.
- G. Li, H. Zhang, D. Fortin, H. Xia and Y. Zhao, Langmuir, 2015, 31, 11709–11716 CrossRef CAS PubMed.
- Y. Fan, W. Zhou, A. Yasin, H. Li and H. Yang, Soft Matter, 2015, 11, 4218–4225 RSC.
- K. Peng, H. Yu, H. Yang, X. Hao, A. Yasin and X. Zhang, Soft Matter, 2017, 13, 2135–2140 RSC.
- L. Wang, Y. Jian, X. Le, W. Lu, C. Ma, J. Zhang, Y. Huang, C. F. Huang and T. Chen, Chem. Commun., 2018, 54, 1229–1232 RSC.
- Z. Li, W. Lu, T. Ngai, X. Le, J. Zheng, N. Zhao, Y. Huang, X. Wen, J. Zhang and T. Chen, Polym. Chem., 2016, 7, 5343–5346 RSC.
- B. Xu, Y. Li, F. Gao, X. Zhai, M. Sun, W. Lu, Z. Cao and W. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 16865–16872 CrossRef CAS PubMed.
- D. H. Gracias, Curr. Opin. Chem. Eng., 2013, 2, 112–119 CrossRef.
- J. Kim, J. A. Hanna, M. Byun, C. D. Santangelo and R. C. Hayward, Science, 2012, 1201, 1200–1205 Search PubMed.
- J. Kim, J. a. Hanna, R. C. Hayward and C. D. Santangelo, Soft Matter, 2012, 8, 2375–2381 RSC.
- E. Palleau, D. Morales, M. D. Dickey and O. D. Velev, Nat. Commun., 2013, 4, 1–7 Search PubMed.
- J. Wang, J. Wang, Z. Chen, S. Fang, Y. Zhu, R. H. Baughman and L. Jiang, Chem. Mater., 2017, 29, 9793–9801 CrossRef CAS.
- X. Peng, T. Liu, Q. Zhang, C. Shang, Q. W. Bai and H. Wang, Adv. Funct. Mater., 2017, 27, 1–8 Search PubMed.
- D. Morales, I. Podolsky, R. W. Mailen, T. Shay, M. D. Dickey and O. D. Velev, Micromachines, 2016, 7, 98 CrossRef PubMed.
- N. Bassik, B. T. Abebe, K. E. Laflin and D. H. Gracias, Polymer, 2010, 51, 6093–6098 CrossRef CAS.
- L. Zhao, J. Huang, Y. Zhang, T. Wang, W. Sun and Z. Tong, ACS Appl. Mater. Interfaces, 2017, 9, 11866–11873 CrossRef CAS PubMed.
- X. Li, X. Cai, Y. Gao and M. J. Serpe, J. Mater. Chem. B, 2017, 5, 2804–2812 RSC.
- J. Kim, C. Kim, Y. S. Song, S. G. Jeong, T. S. Kim and C. S. Lee, Chem. Eng. J., 2017, 321, 384–393 CrossRef CAS.
- I. Tokarev and S. Minko, Soft Matter, 2009, 5, 511–524 RSC.
- M. Dadsetan, Z. Liu, M. Pumberger, C. V. Giraldo, T. Ruesink, L. Lu and M. J. Yaszemski, Biomaterials, 2010, 31, 8051–8062 CrossRef CAS PubMed.
- C. M. Dong and Y. Chen, J. Controlled Release, 2011, 152, e13–e14 CrossRef CAS PubMed.
- H. Li, in Smart Hydrogel Modelling, 2009, pp. 173–218 Search PubMed.
- E. M. White, J. Yatvin, J. B. Grubbs, J. A. Bilbrey and J. Locklin, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1084–1099 CrossRef CAS.
- H. Li, in Smart Hydrogel Modelling, 2009, pp. 295–333 Search PubMed.
- T. Morimoto and F. Ashida, Int. J. Solids Struct., 2015, 56, 20–28 CrossRef.
- B. Xue, M. Qin, T. Wang, J. Wu, D. Luo, Q. Jiang, Y. Li, Y. Cao and W. Wang, Adv. Funct. Mater., 2016, 26, 9053–9062 CrossRef CAS.
- Y. Tan, S. Xu, R. Wu, J. Du, J. Sang and J. Wang, Appl. Clay Sci., 2017, 148, 77–82 CrossRef CAS.
- H. Meng, J. Zheng, X. Wen, Z. Cai, J. Zhang and T. Chen, Macromol. Rapid Commun., 2015, 36, 533–537 CrossRef CAS PubMed.
- K. Zhang, Y. Luo and Z. Li, Soft Matter, 2007, 5, 183–195 CrossRef CAS.
- J. Kim, N. Singh and L. A. Lyon, Angew. Chem., Int. Ed., 2006, 45, 1446–1449 CrossRef CAS PubMed.
- J. Hu, G. Zhang and S. Liu, Chem. Soc. Rev., 2012, 41, 5933–5949 RSC.
- W. C. Liao, S. Lilienthal, J. S. Kahn, M. Riutin, Y. S. Sohn, R. Nechushtai and I. Willner, Chem. Sci., 2017, 8, 3362–3373 RSC.
- D. Men, L. Hang, H. Zhang, X. Zhang, X. Liu, W. Cai and Y. Li, ChemNanoMat, 2018, 4, 165–169 CrossRef CAS.
- L. M. Bonanno and U. A. Delouise, Adv. Funct. Mater., 2010, 20, 573–578 CrossRef CAS PubMed.
- D. Yang, J. Zhao, X. Wang, J. Shi, S. Zhang and Z. Jiang, Biochem. Eng. J., 2017, 117, 52–61 CrossRef CAS.
- H. Liu, L. Rong, B. Wang, R. Xie, X. Sui, H. Xu, L. Zhang, Y. Zhong and Z. Mao, Carbohydr. Polym., 2017, 176, 299–306 CrossRef CAS PubMed.
- J. Park, S. Pramanick, D. Park, J. Yeo, J. Lee, H. Lee and W. J. Kim, Adv. Mater., 2017, 29, 1702859 CrossRef PubMed.
- J. Du, B. Li, C. Li, Y. Zhang, G. Yu, H. Wang and X. Mu, Int. J. Biol. Macromol., 2016, 88, 451–456 CrossRef CAS PubMed.
- Q. Zhu, L. Zhang, K. J. Van Vliet, A. Miserez and N. Holten-Andersen, ACS Appl. Mater. Interfaces, 2018, 10, 10409–10418 CrossRef CAS PubMed.
- C. Gong, S. Shi, P. Dong, B. Kan, M. Gou, X. Wang, X. Li, F. Luo, X. Zhao, Y. Wei and Z. Qian, Int. J. Pharm., 2009, 365, 89–99 CrossRef CAS PubMed.
- M. Prabaharan and J. F. Mano, Macromol. Biosci., 2006, 6, 991–1008 CrossRef CAS PubMed.
- K. K. Westbrook and H. J. Qi, J. Intell. Mater. Syst. Struct., 2007, 19, 597–607 CrossRef.
- C. Yao, Z. Liu, C. Yang, W. Wang, X. J. Ju, R. Xie and L. Y. Chu, Adv. Funct. Mater., 2015, 25, 2980–2991 CrossRef CAS.
- M. E. Harmon, M. Tang and C. W. Frank, Polymer, 2003, 44, 4547–4556 CrossRef CAS.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS.
- S. R. Sershen, G. A. Mensing, M. Ng, N. J. Halas, D. J. Beebe and J. L. West, Adv. Mater., 2005, 17, 1366–1368 CrossRef CAS.
- H. Thérien-Aubin, Z. L. Wu, Z. Nie and E. Kumacheva, J. Am. Chem. Soc., 2013, 135, 4834–4839 CrossRef PubMed.
- W. J. Zheng, N. An, J. H. Yang, J. Zhou and Y. M. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 1758–1764 CrossRef CAS PubMed.
- J. S. Kahn, Y. Hu and I. Willner, Acc. Chem. Res., 2017, 50, 680–690 CrossRef CAS PubMed.
- L. H. Lima, Y. Morales and T. Cabral, Int. J. Retina Vitreous, 2016, 2, 23 CrossRef PubMed.
- P. Patra, A. P. Rameshbabu, D. Das, S. Dhara, A. B. Panda and S. Pal, Polym. Chem., 2016, 7, 5426–5435 RSC.
- H. Warren, M. in het Panhuis, G. M. Spinks and D. L. Officer, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 46–52 CrossRef CAS.
- T. Trongsatitkul and B. M. Budhlall, Polym. Chem., 2013, 4, 1502–1516 RSC.
- T. H. Lee and J. Y. Jho, Macromol. Res., 2018, 26, 659–664 CrossRef CAS.
- R. P. Hamlen, C. E. Kent and S. N. Shafer, Nature, 1965, 206, 1149–1150 CrossRef CAS.
- C. Yang, W. Wang, C. Yao, R. Xie, X. J. Ju, Z. Liu and L. Y. Chu, Sci. Rep., 2015, 5, 1–10 Search PubMed.
- D. Morales, E. Palleau, M. D. Dickey and O. D. Velev, Soft Matter, 2014, 10, 1337–1348 RSC.
- M. Bassil, J. Davenas and M. EL Tahchi, Sens. Actuators, B, 2008, 134, 496–501 CrossRef CAS.
- Z. Liu, R. Tang, D. Xu, J. Liu and H. Yu, Macromol. Rapid Commun., 2015, 36, 1171–1176 CrossRef CAS PubMed.
- Y. H. Chan, M. E. Gallina, X. Zhang, I. C. Wu, Y. Jin, W. Sun and D. T. Chiu, Anal. Chem., 2012, 84, 9431–9438 CrossRef CAS PubMed.
- Y. S. Nam, I. Yoo, O. Yarimaga, I. S. Park, D.-H. Park, S. Song, J.-M. Kim and C. W. Lee, Chem. Commun., 2014, 50, 4251–4254 RSC.
- C. H. Zhu, Y. Lu, J. Peng, J. F. Chen and S. H. Yu, Adv. Funct. Mater., 2012, 22, 4017–4022 CrossRef CAS.
- E. Lee, H. Lee, S. Il Yoo and J. Yoon, ACS Appl. Mater. Interfaces, 2014, 6, 16949–16955 CrossRef CAS PubMed.
- C. Y. Chou, K. S. Chen, W. L. Lin, Y. C. Ye and S. C. Liao, Micromachines, 2017, 8, 5 CrossRef.
- E. Lee, D. Kim, H. Kim and J. Yoon, Sci. Rep., 2015, 5, 1–8 Search PubMed.
- X. Yu, S. Zhou, X. Zheng, T. Guo, Y. Xiao and B. Song, Nanotechnology, 2009, 20, 235702 CrossRef PubMed.
- T. S. Shim, S. H. Kim, C. J. Heo, H. C. Jeon and S. M. Yang, Angew. Chem., Int. Ed., 2012, 51, 1420–1423 CrossRef CAS PubMed.
- W. Gao, L. Wang, X. Wang and H. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 14182–14189 CrossRef CAS PubMed.
- Z. Tang, C. He, H. Tian, J. Ding, B. S. Hsiao, B. Chu and X. Chen, Prog. Polym. Sci., 2016, 60, 86–128 CrossRef CAS.
- M. C. Koetting, J. T. Peters, S. D. Steichen and N. A. Peppas, Mater. Sci. Eng., R, 2015, 93, 1–49 CrossRef PubMed.
- N. S. Nikouei, M. R. Vakili, M. S. Bahniuk, L. Unsworth, A. Akbari, J. Wu and A. Lavasanifar, Acta Biomater., 2015, 12, 81–92 CrossRef CAS PubMed.
- H. Yuk, S. Lin, C. Ma, M. Takaffoli, N. X. Fang and X. Zhao, Nat. Commun., 2017, 8, 1–12 CrossRef PubMed.
- G. Chinga-Carrasco and K. Syverud, J. Biomater. Appl., 2014, 29, 423–432 CrossRef PubMed.
- T. Yokoi, M. Kawashita and C. Ohtsuki, J. Asian Ceram. Soc., 2013, 1, 155–162 CrossRef.
- B. Mishra, Austin J. Biomed. Eng., 2017, 4, 1037 Search PubMed.
- K. U. Jeong, J. H. Jang, D. Y. Kim, C. Nah, J. H. Lee, M. H. Lee, H. J. Sun, C. L. Wang, S. Z. D. Cheng and E. L. Thomas, J. Mater. Chem., 2011, 21, 6824–6830 RSC.
- C. Legros, M. C. De Pauw-Gillet, K. C. Tam, S. Lecommmandoux and D. Taton, Polym. Chem., 2013, 4, 4801 RSC.
- S. V. Wegner, F. C. Schenk, S. Witzel, F. Bialas and J. P. Spatz, Macromolecules, 2016, 49, 4229–4235 CrossRef CAS.
- A. F. Greene, M. K. Danielson, A. O. Delawder, K. P. Liles, X. Li, A. Natraj, A. Wellen and J. C. Barnes, Chem. Mater., 2017, 29, 9498–9508 CrossRef CAS.
- Y. Ito, M. Casolaro, K. Kono and Y. Imanishi, J. Controlled Release, 1989, 10, 195–203 CrossRef CAS.
- D. Shiino, Y. Murata, K. Kataoka, Y. Koyama, M. Yokoyama, T. Okano and Y. Sakurai, Biomaterials, 1994, 15, 121–128 CrossRef CAS PubMed.
- T. Yang, R. Ji, X.-X. Deng, F.-S. Du and Z.-C. Li, Soft Matter, 2014, 10, 2671–2678 RSC.
- J. Cao, S. Liu, Y. Chen, L. Shi and Z. Zhang, Polym. Chem., 2014, 5, 5029–5036 RSC.
- W. L. A. Brooks, G. Vancoillie, C. P. Kabb, R. Hoogenboom and B. S. Sumerlin, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2309–2317 CrossRef CAS.
- F. Horkay, S. H. Cho, P. Tathireddy, L. Rieth, F. Solzbacher and J. Magda, Sens. Actuators, B, 2011, 160, 1363–1371 CrossRef CAS PubMed.
- M. D. Phillips and T. D. James, J. Fluoresc., 2004, 14, 549–559 CrossRef CAS PubMed.
- P. R. Westmark and B. D. Smith, J. Am. Chem. Soc., 1994, 116, 9343–9344 CrossRef CAS.
- T. Kawanishi, M. A. Romey, P. C. Zhu, M. Z. Holody and S. Shinkai, J. Fluoresc., 2004, 14, 499–512 CrossRef CAS PubMed.
- A. Pettignano, S. Grijalvo, M. Häring, R. Eritja, N. Tanchoux, F. Quignard and D. Díaz Díaz, Chem. Commun., 2017, 53, 3350–3353 RSC.
- Y. Dong, W. Wang, O. Veiseh, E. A. Appel, K. Xue, M. J. Webber, B. C. Tang, X. W. Yang, G. C. Weir, R. Langer and D. G. Anderson, Langmuir, 2016, 32, 8743–8747 CrossRef CAS PubMed.
- D. Roy and B. S. Sumerlin, ACS Macro Lett., 2012, 1, 529–532 CrossRef CAS.
- J. C. Athas, C. P. Nguyen, B. C. Zarket, A. Gargava, Z. Nie and S. R. Raghavan, ACS Appl. Mater. Interfaces, 2016, 8, 19066–19074 CrossRef CAS PubMed.
- Z. Sui, W. J. King and W. L. Murphy, Adv. Mater., 2007, 19, 3377–3380 CrossRef CAS.
- P. D. Thornton, R. J. Mart and R. V. Ulijn, Adv. Mater., 2007, 19, 1252–1256 CrossRef.
- Á. Némethy, K. Solti, L. Kiss, B. Gyarmati, M. A. Deli, E. Csányi and A. Szilágyi, Eur. Polym. J., 2013, 49, 1268–1286 CrossRef.
- A. Tamayol, M. Akbari, Y. Zilberman, M. Comotto, E. Lesha, L. Serex, S. Bagherifard, Y. Chen, G. Fu, S. K. Ameri, W. Ruan, E. L. Miller, M. R. Dokmeci, S. Sonkusale and A. Khademhosseini, Adv. Healthcare Mater., 2016, 5, 711–719 CrossRef CAS PubMed.
- A. Shastri, L. M. McGregor, Y. Liu, V. Harris, H. Nan, M. Mujica, Y. Vasquez, A. Bhattacharya, Y. Ma, M. Aizenberg, O. Kuksenok, A. C. Balazs, J. Aizenberg and X. He, Nat. Chem., 2015, 7, 447–454 CrossRef CAS PubMed.
- H. Chen and Y. Lo Hsieh, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 6331–6339 CrossRef CAS.
- J. C. Breger, C. Yoon, R. Xiao, H. R. Kwag, M. O. Wang, J. P. Fisher, T. D. Nguyen and D. H. Gracias, ACS Appl. Mater. Interfaces, 2015, 7, 3398–3405 CrossRef CAS PubMed.
- J. Shang and P. Theato, Soft Matter, 2018, 14, 8401–8407 RSC.
- M. Li, Q. Yang, H. Liu, M. Qiu, T. J. Lu and F. Xu, Small, 2016, 12, 4492–4500 CrossRef CAS PubMed.
- J. Duan, X. Liang, K. Zhu, J. Guo and L. Zhang, Soft Matter, 2017, 13, 345–354 RSC.
- P. Yuan, J. M. McCracken, D. E. Gross, P. V. Braun, J. S. Moore and R. G. Nuzzo, Soft Matter, 2017, 13, 7312–7317 RSC.
- Y. Yajima, M. Yamada, E. Yamada, M. Iwase and M. Seki, Biomicrofluidics, 2014, 8, 024115 CrossRef PubMed.
- M. S. Oh, Y. S. Song, C. Kim, J. Kim, J. B. You, T. S. Kim, C. S. Lee and S. G. Im, ACS Appl. Mater. Interfaces, 2016, 8, 8782–8788 CrossRef CAS PubMed.
- C. L. Randall, E. Gultepe and D. H. Gracias, Trends Biotechnol., 2012, 30, 138–146 CrossRef CAS PubMed.
- K. Kumar, V. Luchnikov, B. Nandan, V. Senkovskyy and M. Stamm, Eur. Polym. J., 2008, 44, 4115–4121 CrossRef CAS.
- J. Pan, S. Yung Chan, J. E. A. Common, S. Amini, A. Miserez, E. Birgitte Lane and L. Kang, J. Biomed. Mater. Res., Part A, 2013, 101, 3159–3169 Search PubMed.
- M. Bae, R. A. Gemeinhart, R. Divan, K. J. Suthar and D. C. Mancini, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2010, 28, C6P24–C6P29 CAS.
- A. Zolfagharian, A. Z. Kouzani, S. Y. Khoo, A. A. A. Moghadam, I. Gibson and A. Kaynak, Sens. Actuators, A, 2016, 250, 258–272 CrossRef CAS.
- A. Zolfagharian, A. Z. Kouzani, B. Nasri-Nasrabadi, S. Adams, S. Yang Khoo, M. Norton, I. Gibson and A. Kaynak, KnE Eng., 2017, 2, 15–22 CrossRef.
- A. Zolfagharian, A. Z. Kouzani, S. Y. Khoo, B. Nasri-Nasrabadi and A. Kaynak, Sens. Actuators, A, 2017, 265, 94–101 CrossRef CAS.
- X. Zhai, Y. Ma, C. Hou, F. Gao, Y. Zhang, C. Ruan, H. Pan, W. W. Lu and W. Liu, ACS Biomater. Sci. Eng., 2017, 3, 1109–1118 CrossRef CAS.
- S. E. Bakarich, R. Gorkin, M. In Het Panhuis and G. M. Spinks, Macromol. Rapid Commun., 2015, 36, 1211–1217 CrossRef CAS PubMed.
- B. P. Lee and S. Konst, Adv. Mater., 2014, 26, 3415–3419 CrossRef CAS PubMed.
- A. B. Baker, D. F. Wass and R. S. Trask, Sens. Actuators, B, 2018, 254, 519–525 CrossRef CAS.
- A. H. Velders, J. A. Dijksman and V. Saggiomo, Appl. Mater. Today, 2017, 9, 271–275 CrossRef.
- Y. S. Kim, M. Liu, Y. Ishida, Y. Ebina, M. Osada, T. Sasaki, T. Hikima, M. Takata and T. Aida, Nat. Mater., 2015, 14, 1–6 CrossRef PubMed.
- K. Yoshida, S. Nakajima, R. Kawano and H. Onoe, Sens. Actuators, B, 2018, 272, 361–368 CrossRef CAS.
- X. Liu, T.-C. Tang, E. Tham, H. Yuk, S. Lin, T. K. Lu and X. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 2200–2205 CrossRef CAS PubMed.
- E. Wang, M. S. Desai and S. Lee, Nano Lett., 2013, 13, 2826–2830 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |