Trends and targets in antiviral phototherapy
Abstract
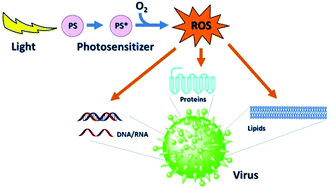
Photodynamic therapy (PDT) is a well-established treatment option in the treatment of certain cancerous and pre-cancerous lesions. Though best-known for its application in tumor therapy, historically the photodynamic effect was first demonstrated against bacteria at the beginning of the 20th century. Today, in light of spreading antibiotic resistance and the rise of new infections, this photodynamic inactivation (PDI) of microbes, such as bacteria, fungi, and viruses, is gaining considerable attention. This review focuses on the PDI of viruses as an alternative treatment in antiviral therapy, but also as a means of viral decontamination, covering mainly the literature of the last decade. The PDI of viruses shares the general action mechanism of photodynamic applications: the irradiation of a dye with light and the subsequent generation of reactive oxygen species (ROS) which are the effective phototoxic agents damaging virus targets by reacting with viral nucleic acids, lipids and proteins. Interestingly, a light-independent antiviral activity has also been found for some of these dyes. This review covers the compound classes employed in the PDI of viruses and their various areas of use. In the medical area, currently two fields stand out in which the PDI of viruses has found broader application: the purification of blood products and the treatment of human papilloma virus manifestations. However, the PDI of viruses has also found interest in such diverse areas as water and surface decontamination, and biosafety.
- This article is part of the themed collections: Coronavirus articles - free to access collection, 2019 Perspective article collection and Nano- and Molecular Engineering of Photosensitisers


 Please wait while we load your content...
Please wait while we load your content...