Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
DOI: 10.1039/C9OB90139F
(Correction)
Org. Biomol. Chem., 2019, 17, 8259-8260
Correction: cis or trans with class II diterpene cyclases
Meirong
Jia
and
Reuben J.
Peters
*
Roy J. Carver Department of Biochemistry, Biophysics & Molecular Biology, Iowa State University, Ames, IA 50010, USA. E-mail: rjpeters@iastate.edu
Received
14th August 2019
, Accepted 14th August 2019
First published on 21st August 2019
Abstract
Correction for ‘cis or trans with class II diterpene cyclases’ by Meirong Jia, et al., Org. Biomol. Chem., 2017, 15, 3158–3160.
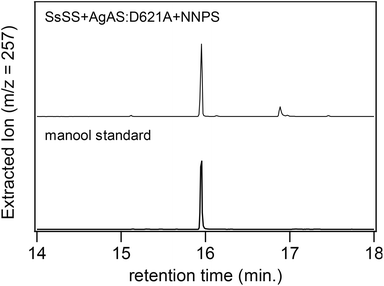
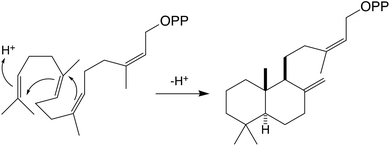
Further investigation indicates that our assignment of the product resulting from AgAS:D621A reacting with the alternative substrate (Z,Z,Z)-nerylneryl diphosphate (NNPP, 2), produced by a previously identified NNPP synthase (NNPS),1 was incorrect (page 3160 of print/pdf of the article). Specifically, upon further reacting this product with the subsequently acting sclareol synthase from Salvia sclarea (SsSS), which promiscuously reacts with a side variety of such precursors, catalyzing heterolytic lysis of the allylic diphosphate ester and addition of water to the ensuing tertiary carbocation to yield a tertiary hydroxyl derivative,2,3 the final product was found to be manool (Fig. 1). This was further verified by NMR, with polarized optical spectra demonstrating that this manool was of ‘normal’ (+) configuration (data not shown). Given that manool is produced by SsSS from normal labda-8(17),13E-dienyl diphosphate,2 and the configuration of the carbon–carbon double bond at carbon-13 (C13) is lost during the SsSS catalyzed reaction, this result strongly indicates that AgAS:D621A reacts with NNPP to produce normal labda-8(17),13Z-dienyl diphosphate in which the produced decalin bicycle has trans substituents across the C5–C10 bridgehead carbons. Accordingly, Scheme 2 in the published Communication should be replaced with Scheme 1 shown here. The authors apologize for this erroneous assignment.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- J. Zi, Y. Matsuba, Y. Hong, A. Jackson, E. Pichersky, D. J. Tantillo and R. J. Peters, J. Am. Chem. Soc., 2014, 136, 16951–16953 CrossRef CAS PubMed.
- M. Jia, K. C. Potter and R. J. Peters, Metab. Eng., 2016, 37, 24–34 CrossRef CAS PubMed.
- M. Jia, S. K. Mishra, S. Tufts, R. L. Jernigan and R. J. Peters, Metab. Eng., 2019, 55, 44–58 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |