Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Enantioselective copper catalysed intramolecular C–H insertion reactions of α-diazo-β-keto sulfones, α-diazo-β-keto phosphine oxides and 2-diazo-1,3-diketones; the influence of the carbene substituent
Amy E.
Shiely
a,
Catherine N.
Slattery
a,
Alan
Ford
a,
Kevin S.
Eccles
a,
Simon E.
Lawrence
a and
Anita R.
Maguire
*b
aDepartment of Chemistry, Analytical and Biological Chemistry Research Facility, University College Cork, Cork, Ireland
bDepartment of Chemistry and School of Pharmacy, Analytical and Biological Chemistry Research Facility, Synthesis and Solid State Pharmaceutical Centre, University College Cork, Cork, Ireland. E-mail: a.maguire@ucc.ie
First published on 17th January 2019
Abstract
Correction for ‘Enantioselective copper catalysed intramolecular C–H insertion reactions of α-diazo-β-keto sulfones, α-diazo-β-keto phosphine oxides and 2-diazo-1,3-diketones; the influence of the carbene substituent’ by Amy E. Shiely et al., Org. Biomol. Chem., 2017, 15, 2609–2628.
The authors regret that there were errors in Fig. 3. Specifically, the bars representing ligands 15 and 16 were incorrectly labelled in the legend and there was an error in one of the values used to prepare the figure. The correct figure prepared using the data from Table 1 is shown below.
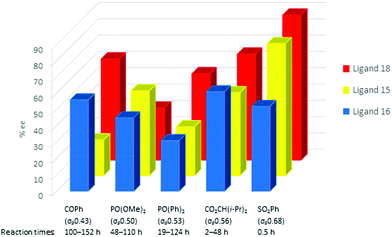 | ||
| Fig. 3 Impact of variation of the electron withdrawing group on the enantioselectivity with ligands 15, 16 and 18. | ||
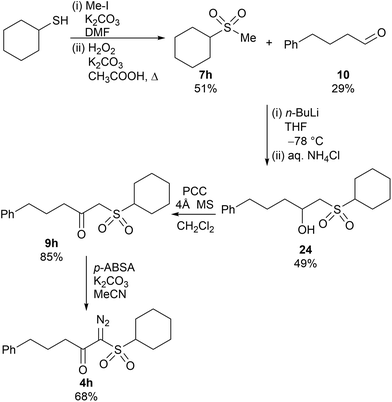
In addition, there was an error Scheme 5 where the diazo group was missing from the final product 4h. The correct scheme is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |