Regio- and chemo-selective cyclization of allenic-Ugi products for the synthesis of 3-pyrroline skeletons†‡
Abstract
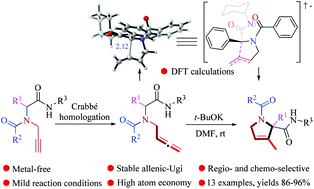
A highly efficient and stable novel class of allenic-Ugi products through a Crabbé homologation reaction is successfully demonstrated. Then, a regio- and chemo-selective cyclization of allenic-Ugi derivatives in a 5-exo-dig fashion to access 3-pyrroline skeletons is developed. Also, computational studies were performed and explained to provide insights into the reaction mechanism. This approach displays high bond-forming efficiency and atom economy with high yields.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...