The mechanism and structure–activity relationship of amide bond formation by silane derivatives: a computational study†
Abstract
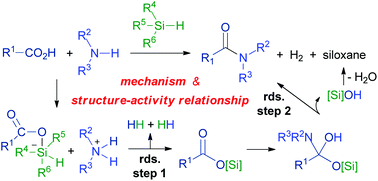
The condensation of carboxylic acids and amines mediated by silane derivatives provided a straightforward and sustainable method for amide bond formation with minimal waste. However, the detailed mechanism and structure–activity relationship of substrates, the topics that are of interest for both academic and industrial applications, were not clear. Herein, a systematic computational study was conducted to solve the two questions. We found that the two previously proposed mechanisms involving intramolecular acyl transfer or silanolate were less likely because the associated silanone intermediate and zwitterion adducts were too unstable with higher overall energy barriers. By comparison, the mechanism involving deprotonation of carboxylic acids, addition of carboxylates on silane reagents, dihydrogen formation to afford an acyloxysilane intermediate, carboxylic-acid-assisted addition of amines, and concerted proton transfer/amide formation, was found to be more favorable with overall energy barriers varying between 24 and 28 kcal mol−1 for the different calculated cases. Meanwhile, the dihydrogen formation and amide formation processes are both potential rate-determining steps. Energy composition, atomic charge, and distortion–interaction analyses indicated that the steric effect of silane reagents was more important than the electronic effect, making less bulky silane reagents more reactive. On the other hand, the dihydrogen formation process was mainly controlled by the electronic effect of the substituents of carboxylic acids and amines while the amide formation process was mainly influenced by their steric effect. As a result, less bulky, less acidic alkyl carboxylic acids are more reactive than unsaturated carboxylic acids, and less bulky, medium basic primary alkyl amines are more reactive than secondary alkyl amines and primary aryl amines. The related results provided deeper mechanistic insights into the amide bond formation mediated by silane derivatives and can act as a reference for further experimental design.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...