Unified synthesis and cytotoxic activity of 8-O-methylfusarubin and its analogues†
Abstract
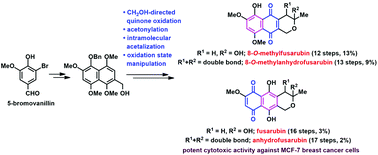
A simple and unified synthesis of four related pyranonaphthoquinone natural products, e.g. 8-O-methylfusarubin, 8-O-methylanhydrofusarubin, fusarubin and anhydrofusarubin, is reported. The key synthetic features include the precedented Diels–Alder cycloaddition to assemble the naphthalene skeleton, selective formylation and acetonylation and intramolecular acetalization to construct the pyran ring. Manipulation of the oxidation state of the naphthoquinone core was performed to construct the two analogues, fusarubin and anhydrofusarubin. This work also highlights an unprecedented directing effect of the hydroxymethylene group in the selective hypervalent iodine-mediated quinone oxidation. The four synthetic compounds were evaluated for their in vitro cytotoxic activities against six human cancer cells. 8-O-Methylfusarubin was the most potent analogue and displayed excellent cytotoxic activity against MCF-7 breast cancer cells with an IC50 value of 1.01 μM with no cytotoxic effect on noncancerous Vero cells, which could potentially be a promising lead compound for anti-breast cancer drug discovery.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...