Organocatalytic asymmetric synthesis of dihydrofuran-spirooxindoles from benzylidene malononitriles and dioxindoles†
Abstract
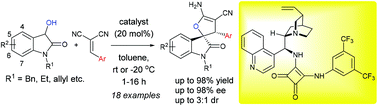
The first organocatalytic enantioselective synthesis of dihydrofuran-spirooxindoles having a linkage at the 2-position of the dihydrofuran motif has been developed. Dioxindoles and benzylidene malononitriles were employed in this method. The desired spirooxindole products were obtained via a Michael reaction followed by a Pinner reaction and isomerization and good to high yields with moderate diastereo and good to high enantioselectivities were observed.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...