Open Access Article
Open Access ArticleSynthesis of α-CF3-proline derivatives by means of a formal (3 + 2)-cyclisation between trifluoropyruvate imines and Michael acceptors†
Michael
Winter
a,
Kirill
Faust
b,
Markus
Himmelsbach
 c and
Mario
Waser
c and
Mario
Waser
 *a
*a
aInstitute of Organic Chemistry, Johannes Kepler University Linz, Altenbergerstr. 69, 4040 Linz, Austria. E-mail: Mario.waser@jku.at
bInstitute of Catalysis, Johannes Kepler University Linz, Altenbergerstr. 69, 4040 Linz, Austria
cInstitute of Analytial Chemistry, Johannes Kepler University Linz, Altenbergerstr. 69, 4040 Linz, Austria
First published on 24th May 2019
Abstract
We herein report the first formal (3 + 2)-cyclisation between 3,3,3-trifluoropyruvate-derived imines and indandione-based Michael acceptors. This reaction gives access to a novel class of spirocyclic α-CF3-α-proline derivatives with complete control of the diastereoselectivity under phase transfer-catalysed reaction conditions.
Introduction
The synthesis of fluorine-containing α-amino acids became a heavily investigated topic over the last years.1–3 The large interest in the hereby accessed fluorinated amino acids is based on the fact that the incorporation of fluorine-containing α- or β-amino acids into peptides and proteins heavily effects their (bio)-chemical and (bio)-physical properties.3 One particularly appealing approach to alter the nature of biologically active molecules is the introduction of a trifluoromethyl group.4,5 A broad variety of different synthesis strategies to install either a single fluorine atom, or a polyfluorinated group have been developed in the past.6–9 Concerning the synthetic introduction of the trifluoromethyl group, one could either rely on a nucleophilic8 or electrophilic7 trifluoromethylation of a given compound, or, alternatively, one uses simple CF3-containing building blocks to access further structural complexity. One commercially readily available CF3-starting material is ethyl 3,3,3-trifluoropyruvate (1), which has been commonly used for further (asymmetric) transformations in the past.10–12 Hereby the use of (protected or unprotected) trifluoropyruvate-derived imines is an especially interesting strategy to access (chiral) α-trifluoromethylated α-amino acids.11 Very interestingly, pyruvate 1-based imines have recently also been used as dipolarophiles for formal (3 + 2)-cyclisations upon reactions with suited 1,3-dipolar compounds.12 Alternatively, besides their use as dipolarophiles in (3 + 2)-annulations, imines can also serve as precursors for the in situ generation of 1,3-dipolar azomethine ylides or 2-azaallyl anions, which then may react with suited dipolarophiles in a (3 + 2)-fashion.13 In this regard also the use of CF3-containing imines for the construction of 2-CF3-substituted pyrrolidines has been described.14 Very interestingly however, and to the best of our knowledge, the trifluoropyruvate-based benzylic imines 215 or the isomeric imines 3 have so far not been used as precursors for the in situ generation an utilisation of 1,3-dipolar species. This comes as a surprise, as an (3 + 2)-annulation reaction of 2-azaallyl anions A with suited (Michael) acceptors B would give access to α-CF3-containing α-proline derivatives 4 or 5 straightforwardly (Scheme 1). Spurred by the recent progress in the use of in situ generated 2-azaallyl anions for (enantioselective) C–C bond forming reactions (often involving a formal Umpolung of the inherent starting imine reactivity),16–18 we became interested in investigating the potential of trifluoropyruvate-based benzylic imines 2 for formal (3 + 2)-cyclisations with different Michael acceptors to access a novel class of α-CF3-substituted α-proline derivatives 4 (via γ-attack of the starting imine) or 5 (via Umpolung of the initial imine 2). | ||
| Scheme 1 Targeted use of trifluoropyruvate 1-based imines to access α-CF3-α-proline derivatives 4 and/or 5. | ||
Results and discussion
The formation of benzylic imines 2 from pyruvate 1 was investigated in the past, but the synthetic use of these imines has so far only sparingly been evaluated.19 In these initial reports, Soloshonok and co-workers also found that isolation of imines 2 is hardly possible, as these compounds tend to undergo a very rapid double bond migration to the isomeric benzaldimines 3 under basic and/or higher temperature reaction conditions.19 Keeping this observation in mind, we reasoned that the α-position of the corresponding 2-azaallyl anions (that will be obtained under basic reaction conditions) will be the preferred nucleophilic site, which should then provide a route towards proline derivatives 5, rather than 4.We started our investigations by carrying out the reaction between the phenyl-based imine 3a (obtained directly from 1 upon reaction with benzylamine)19,20 and the benzylidene indandione 6a as the acceptor21 (Table 1 gives an overview of the most significant results obtained in this screening). Already our initial experiments showed that the targeted (3 + 2)-annulation is possible, giving the α-attack-derived spiro compound 5a as the sole cyclization product. It should be noted that in neither case any formation of the corresponding γ-addition product 4 or any classical Michael addition product were observed. As expected, the presence of a simple ammonium salt phase-transfer catalyst (PTC) significantly improved the conversion (compare entries 1 and 2) and the reaction proceeded with full conversion and a promising initial yield and diastereoselectivity in the presence of a catalytic amount of aqueous base (entry 2). In order to improve yield and dr we next screened different bases (in all these cases CH2Cl2 was used as the solvent, but we of course also tested other solvents like toluene or THF later on, which however did not allow for better results!).
| Entrya | Cat.b | Base | Conv. (%)c | Yieldd (%) | drc |
|---|---|---|---|---|---|
| a Unless otherwise stated all reactions were carried out by stirring a mixture of 0.1 mmol 3a and 1.2 equiv. 6a with 10 mol% of catalyst in the presence of base in CH2Cl2 at room temperature for 24 hours. b THAB (tetrahexylammonium bromide), TEBAB (benzyltriethylammonium bromide). c Determined by 1H and 19F NMR of the crude product. d Isolated yields. e Using 20 mol% TEBAB. f Using 1 mmol 3a and 2 equiv. 6a. g Using 30 mol% TEBAB or the analogous chloride TEBAC. | |||||
| 1 | — | KOH (50%) | 15 | n.d. | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (0.1 eq.) | |||||
| 2 | THAB | KOH (50%) | >99 | 57 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| (0.1 eq.) | |||||
| 3 | THAB | KOH (50%) | 91 | 74 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| (1 eq.) | |||||
| 4 | THAB | KOH (s) | 93 | 72 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (1 eq.) | |||||
| 5 | THAB | Cs2CO3 (s) | >99 | 79 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (1 eq.) | |||||
| 6 | THAB | K3PO4 (s) | 95 | 51 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| (1 eq.) | |||||
| 7 | THAB | LiOH (s) | 90 | 68 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (1 eq.) | |||||
| 8 | TEBAB | LiOH (s) | >99 | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (1 eq.) | |||||
| 9 | TEBAB | LiOH (s) | >99 | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (2 eq.) | |||||
| 10 | TEBABe | LiOH (s) | >99 | 75 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (2 eq.) | |||||
| 11f | TEBABg | LiOH (s) | >99 | 98 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| (3 eq.) | |||||
By increasing the amount of base, we found that solid KOH allows for a slightly higher diastereoselectivity than aqueous KOH (entries 3 and 4). When using different solid bases (entries 4–7) it turned out that LiOH allows for complete control of the diastereoselectivity combined with a reasonable isolated yield of 68% (entry 7). When testing other simple ammonium salt PTCs we found that benzyltriethylammonium bromide performs more or less similarly as tetrahexylammonium bromide (entries 7 and 8) and we later on also realized that the corresponding ammonium chlorides allow for the same outcome. In order to improve the yield further, we increased the amount of base and catalyst (entries 9–11) and finally used a twofold excess of the acceptor 6a combined with three equivalents of solid LiOH in the presence of 30 mol% ammonium salt catalyst, which gave 5a in literally quantitative isolated yield on 1 mmol reaction scale (entry 9).
The relative configuration of the major diastereomer was first investigated by NOESY NMR experiments. Here we observed NOEs between the two benzylic protons (substantiating a cis-configuration of the two phenyl rings) as well as a weak NOE between the ester group and one phenyl group (supporting a cis relationship between the ester and the phenyls as well).20 We later on were able to carry out single crystal X-ray analysis of the bromo-derivative 5b22 (compare with Scheme 3), which unambiguously proved the same relative configuration for that derivative as well, supporting our earlier NOESY results.
Having identified high yielding and highly diastereoselective racemic phase transfer-catalysed conditions for the (3 + 2)-cyclisation between 3a and 6a, we next screened a variety of different chiral ammonium salt phase transfer catalysts under different conditions for this reaction.20,23 Unfortunately however, all these efforts were rather disappointing, as we were not able to obtain any reasonable enantioselectivity for this reaction. Scheme 2 gives the best result obtained with a Cinchona alkaloid-based chiral phase transfer catalyst, but a variety of other catalyst systems under different conditions were tested as well, but none of them gave any satisfying selectivity (as shown in the online ESI†).20
As we were not successful in developing a satisfyingly enantioselective protocol, we investigated the application scope for the reaction between imines 3 and 1,3-indandiones 6 using achiral ammonium salt PTCs only (Scheme 3).
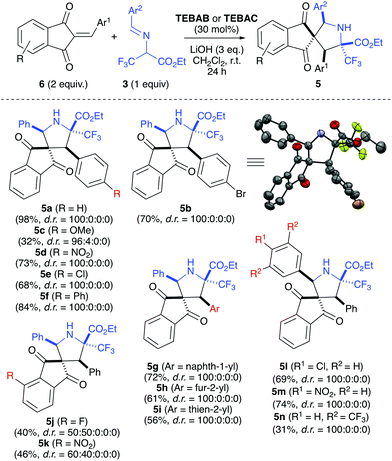 | ||
| Scheme 3 Application scope of the diastereoselective (3 + 2)-cyclisation of imines 3 and acceptors 6. | ||
A variety of acceptors with different Ar1-groups were tolerated well (giving products 5a–5i). Only the methoxy-containing 5c was obtained in a lower yield, mainly because of a significantly slower conversion in that specific case. As mentioned above, 5b could be analysed by single crystal X-ray analysis and the relative configuration of the other products was assigned in analogy. When using alternative Ar2-groups on the donor side, the products 5l and 5m were obtained in similar yields and with the same high diastereoselectivity. Surprisingly, the bis-CF3-containing 5n was obtained in a lower yield only, mainly because of the accompanying formation of unidentified side-products (most probably originating from background hydrolysis of the starting imine). Unfortunately, the introduction of other substituents on the indandione side resulted in slightly reduced yields (see products 5j and 5k). Furthermore, these products were also obtained as mixtures of diastereomers (differing in the configuration of the spiro carbon). The fact that the configuration of this additional stereogenic center was not controlled in this otherwise highly diastereoselective reaction came as a surprise, and unfortunately we were not able to overcome this issue.
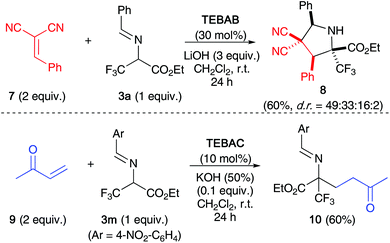
Finally, we also tested if other Michael acceptors may be used for this (3 + 2)-cyclisation with imines 3. As shown in Scheme 4, the arylidene malonitrile 7 gave the (3 + 2)-product 8 as well, but with very low diastereoselectivity and in this case also significant amounts of different not identified impurities were formed. In sharp contrast, methyl vinyl ketone 9 was the only acceptor so far that did not give the cyclisation product, but rather underwent a reasonably high yielding Michael addition when reacted with the NO2-containing imine 3m (the same conjugated addition reaction, but significantly slower, was observed with the phenyl-based 3a).
Conclusions
The reaction between 3,3,3-trifluoropyruvate (1)-derived imines 3 and indandione-based Michael acceptors 6 allows for a formal (3 + 2)-cyclisation which gives access to an unprecedented class of α-CF3-α-proline derivatives 5. By choice of the proper phase transfer-catalysed reaction conditions (i.e. the used base) this reaction performs with literally complete diastereoselectivity. Unfortunately, the use of chiral ammonium salt catalysts turned out to be not satisfying so far. Other acceptors were tested as well and arylidene malonitrile 7 undergoes this type of (3 + 2)-cyclization as well, but with relatively poor diastereoselectivity, while methyl vinylketone (9) acted as a Michael acceptor only.Experimental details20
General procedure
The arylidene indandiones 6a–k (0.2 mmol) were dissolved in dry CH2Cl2 (2 mL), followed by the addition of LiOH (0.3 mmol, 7.2 mg), TEBAC (30 mol%, 0.03 mmol, 7.8 mg) and imines 3a–d (0.1 mmol). The reaction mixture was stirred for 24 h at room temperature followed by filtration over a pad of silica and washing with Et2O. After evaporation of the solvent, the product was purified by column chromatography with a gradient of heptanes and EtOAc (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10
1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield products 5a–n in the reported yields and diastereomeric ratios.
1) to yield products 5a–n in the reported yields and diastereomeric ratios.
Analytical data for 5a (this compound was also synthesised on 1 mmol scale)
1H NMR (300 MHz, CDCl3, 298 K) δ 7.76–7.73 (m, 1H), 7.60–7.52 (m, 3H), 7.15–7.06 (m, 10H), 4.93–4.83 (m, 2H), 4.65 (s, 1H), 4.31–4.20 (m, 1H), 3.96–3.86 (m, 1H), 0.94 (t, J = 7.1 Hz, 3H) ppm; 13C NMR (176 MHz, CDCl3, 298 K) δ 199.4, 199.4, 166.7, 142.6, 142.1, 135.9, 135.6, 133.5, 133.5, 130.3, 128.4, 128.4, 128.2, 127.9, 126.7, 126.2 (q, J = 298 Hz), 124.1, 122.0, 122.9, 74.6 (q, J = 28 Hz), 72.1, 71.3, 63.0, 60.7, 14.2, 13.4 ppm; 19F-NMR (282 MHz, CDCl3, 298 K): δ = −74.22 (s, 3F) ppm; HRMS (ESI): m/z calcd for C28H22F3NO4 [M + H]+ = 494.1574, found: 494.1580.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was generously supported by the Austrian Science Funds (FWF): Project No. P30237 (financed by the Austrian National Foundation for Research, Technology and Development and Research Department of the State of Upper Austria). The used NMR spectrometers were acquired in collaboration with the University of South Bohemia (CZ) with financial support from the European Union through the EFRE INTERREG IV ETC-AT-CZ program (project M00146, “RERI-uasb”).Notes and references
- V. P. Kukhar and V. A. Soloshonok, in Fluorine Containing Amino Acids: Synthesis and Properties, Wiley, New York, 1995 Search PubMed.
- (a) X.-L. Qiu, W.-D. Meng and F.-L. Qing, Tetrahedron, 2004, 60, 6711 CrossRef CAS; (b) X.-L. Qiu and F.-L. Qing, Eur. J. Org. Chem., 2011, 3261 CrossRef CAS; (c) R. Smits, C. D. Cadicamo, K. Burger and B. Koksch, Chem. Soc. Rev., 2008, 37, 1727 RSC; (d) J. L. Acena, A. Simon-Fuentes and S. Fustero, Curr. Org. Chem., 2010, 14, 928 CrossRef CAS; (e) T. L. March, M. R. Johnston, P. J. Duggan and J. Gardiner, Chem. Biodiversity, 2012, 9, 2410 CrossRef CAS PubMed; (f) A. M. Remete, M. Nonn, S. Fustero, F. Fülöp and L. Kiss, Tetrahedron, 2018, 74, 6367 CrossRef CAS.
- (a) N. C. Yoder and K. Jumar, Chem. Soc. Rev., 2002, 31, 335 RSC; (b) G. Akcay and K. Kumar, J. Fluorine Chem., 2009, 130, 1178 CrossRef CAS PubMed; (c) M. Salwiczek, E. K. Nyakatura, U. I. M. Gerling, S. Ye and B. Koksch, Chem. Soc. Rev., 2012, 41, 2135 RSC; (d) E. N. G. Marsh, Acc. Chem. Res., 2014, 47, 2878 CrossRef CAS PubMed; (e) A. A. Berger, J.-S. Völler, N. Budisa and B. Koksch, Acc. Chem. Res., 2017, 50, 2093 CrossRef CAS PubMed; (f) S. Huhmann and B. Koksch, Eur. J. Org. Chem., 2018, 3667 CrossRef CAS.
- Y. Zhou, j. Wang, Z. gu, S. Wang, W. Zhu, J. L. Acena, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed.
- R. Smits, C. D. Cadicamo, K. Burger and B. Koksch, Chem. Soc. Rev., 2008, 37, 1727 RSC.
- (a) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (b) X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed.
- For illustrative reviews on electrophilic trifluoromethylations: (a) S. Barata-Vallejo, B. Lantano and A. Postigo, Chem. – Eur. J., 2014, 20, 16806 CrossRef CAS PubMed; (b) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed; (c) T. Umemoto, in Modern Synthesis Processes and Reactivity of Fluorinated Compounds – Progress in Fluorine Science, ed. H. Groult, F. R. Leroux and A. Tressaud, Elsevier, 2017, pp. 265–287 Search PubMed.
- For selected reviews on nucleophilic trifluoromethylations: (a) B. R. Langlois, T. Billard and S. Roussel, J. Fluorine Chem., 2005, 126, 173 CrossRef CAS; (b) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed.
- X.-H. He, Y.-L. Ji, C. Peng and B. Han, Adv. Synth. Catal., 2019, 361, 1923 CrossRef CAS.
- For selected recent asymmetric examples using trifluoropyruvate: (a) K. Fujity and K. Mikami, J. Fluorine Chem., 2019, 219, 50 CrossRef; (b) Y. Tang, J. Xu, J. Yang, L. Lin, X. Feng and X. Liu, Chem., 2018, 4, 1658 CrossRef CAS; (c) T. P. Le, K. Higashita, S. Tanaka, M. Yoshimura and M. Kitamura, Org. Lett., 2018, 20, 7149 CrossRef CAS PubMed; (d) K. Aikawa, K. Yabuuchi, K. Torii and K. Mikami, Beilstein J. Org. Chem., 2018, 14, 576 CrossRef CAS PubMed; (e) B.-B. Huang, L. Wu, R.-R. Liu, L.-L. Xing, R.-X. Liang and Y.-X. Jia, Org. Chem. Front., 2018, 5, 929 RSC.
- For selected recent asymmetric examples using trifluoropyruvate-based imines: (a) M. Sawa, K. Morisaki, Y. Kondo, H. Morimoto and T. Ohshima, Chem. – Eur. J., 2017, 23, 17022 CrossRef CAS PubMed; (b) R. Yonesaki, Y. Kondo, W. Akkad, M. Sawa, K. Morisaki, H. Morimoto and T. Ohshima, Chem. – Eur. J., 2018, 24, 15211 CrossRef CAS PubMed; (c) M. Frias, A. C. Carrasco, A. Fraile and J. Aleman, Chem. – Eur. J., 2018, 24, 3117 CrossRef CAS PubMed; (d) K. Morisaki, M. Sawa, R. Yonesaki, H. Morimoto, K. Mashima and T. Ohshima, J. Am. Chem. Soc., 2016, 138, 6194 CrossRef CAS PubMed.
- (a) Y. V. Rassukana, L. V. Bezgubenko, O. V. Stanko, E. B. Rusanov, I. B. Kulik and P. P. Onys'ko, Tetrahedron: Asymmetry, 2017, 28, 555 CrossRef CAS; (b) L. Wei, Q.-H. Li and C.-J. Wang, J. Org. Chem., 2018, 83, 11814 CrossRef CAS PubMed; (c) A. Sanz-Vidal, J. Miro, M. Sanchez-Rosello, C. del Pozo and S. Fustero, J. Org. Chem., 2016, 81, 6515 CrossRef CAS PubMed.
- (a) C. Najera and J. M. Sansano, Curr. Org. Chem., 2003, 7, 1105 CrossRef CAS; (b) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765 CrossRef CAS PubMed; (c) J. Adrio and J. C. Carretero, Chem. Commun., 2011, 47, 6784 RSC; (d) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed; (e) A. G. Meyer and J. H. Ryan, Molecules, 2016, 21, 935 CrossRef PubMed.
- For selected recent examples: (a) M. Ma, Y. Zhu, Q. Sun, X. Li, J. Su, L. Zhao, Y. Zhao, S. Qiu, W. Yan, K. Wang and R. Wang, Chem. Commun., 2015, 51, 8789 RSC; (b) Q. Sun, X. Li, J. Su, L. Zhao, M. Ma, Y. Zhu, Y. Zhao, R. Zhu, W. Yan, K. Wang and R. Wang, Adv. Synth. Catal., 2015, 357, 3187 CrossRef CAS; (c) A. Ponce, I. Alonso, J. Adrio and J. C. Carretero, Chem. – Eur. J., 2016, 22, 4952 CrossRef CAS PubMed; (d) Z. Dong, Y. Zhu, B. Li, C. Wang, W. Yan, K. Wang and R. Wang, J. Org. Chem., 2017, 82, 3482 CrossRef CAS PubMed; (e) Q. Liu, K. Zhao, Y. Zhi, G. Raabe and D. Enders, Org. Chem. Front., 2017, 4, 1416 RSC; (f) H.-Z. Gui, Y.-N. Gao, Y. Wei and M. Shi, Chem. – Eur. J., 2018, 24, 10038 CrossRef CAS PubMed; (g) Y. You, W.-Y. Lu, Z.-H. Wang, Y.-Z. Chen, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2018, 20, 4453 CrossRef CAS PubMed; (h) B. Li, J. Liu, F. Gao, M. Sun, Y. Guo, Y. Zhou, D. Wen, Y. Deng, H. Chen, K. Wang and W. Yan, Org. Biomol. Chem., 2019, 17, 2892 RSC; (i) B. Li, F. Gao, X. Feng, M. Sun, Y. Guo, D. Wen, Y. Deng, J. Huang, K. Wang and W. Yan, Org. Chem. Front., 2019, 6, 1567 RSC.
- For the addition of C-nucleophiles to imines 2 and subsequent α-CF3-proline formation: (a) M. Eckert, S. Moulin, F. Monnier, I. D. Titanyuk, S. N. Osipov, T. Roisnel, S. Derien and P. H. Dixneuf, Chem. – Eur. J., 2011, 17, 9456 CrossRef CAS PubMed; (b) G. Huang, Z. Yin and X. Zhang, Chem. – Eur. J., 2013, 19, 11992 CrossRef CAS PubMed; (c) G. Chaume, M.-C. van Severen, S. Marinkovic and T. Brigaud, Org. Lett., 2016, 8, 6123 CrossRef PubMed.
- For overviews on imine Umpolung: (a) D. Seebach and D. Enders, Angew. Chem., Int. Ed. Engl., 1975, 14, 15 CrossRef; (b) J. Novacek and M. Waser, Angew. Chem., Int. Ed., 2015, 54, 14228 CrossRef PubMed.
- (a) M. Liu, J. Li, X. Xiao, Y. Xie and Y. Shi, Chem. Commun., 2013, 49, 1404 RSC; (b) Y. Wu, L. Hu, Z. Li and L. Deng, Nature, 2015, 523, 445 CrossRef CAS PubMed; (c) L. Hu, Y. Wu, Z. Li and L. Deng, J. Am. Chem. Soc., 2016, 138, 15817 CrossRef CAS PubMed; (d) P. Chen, Z. Yue, J. Zhang, X. Lv, L. Wang and J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 13316 CrossRef CAS PubMed; (e) Y.-L. Su, Y.-H. Li, Y.-G. Chen and Z.-Y. Han, Chem. Commun., 2017, 53, 1985 RSC; (f) Y. Yoshida, T. Mino and M. Sakamoto, Chem. – Eur. J., 2017, 23, 12749 CrossRef CAS PubMed; (g) B. Hu and L. Deng, Angew. Chem., Int. Ed., 2018, 57, 2233 CrossRef CAS PubMed; (h) Y. Yoshida, Y. Moriya, T. Mino and M. Sakamoto, Adv. Synth. Catal., 2018, 360, 4142 CrossRef CAS.
- (3 + 2)-cyclizations: (a) X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 13819 CrossRef CAS PubMed; (b) C. Guo, J. Song and L.-Z. Gong, Org. Lett., 2013, 15, 2676 CrossRef CAS PubMed; (c) L. Tian, X.-Q. Hu, Y.-H. Li and P.-F. Xu, Chem. Commun., 2013, 49, 7213 RSC.
- (a) V. A. Soloshonok and V. P. Kukhar, Tetrahedron, 1997, 53, 9307 CrossRef; (b) H. Ohkura, D. O. Berbasov and V. A. Soloshonok, Tetrahedron, 2003, 59, 1647 CrossRef CAS.
- Further details can be found in the online ESI.†.
- For selected recent reports describing the use of compounds 6 for (3 + 2)-cyclizations: (a) Y.-R. Chen, M. R. Ganapuram, K.-H. Hsieh, K.-H. Chen, P. Karanam, S. S. Vagh, Y.-C. Liou and W. Lin, Chem. Commun., 2018, 54, 12702 RSC; (b) J.-K. Yu, H.-W. Chien, Y.-J. Lin, P. Karanam, Y.-H. Chen and W. Lin, Chem. Commun., 2018, 54, 9921 RSC; (c) Y. Huang, J. Sun and C.-G. Yan, ChemistrySelect, 2017, 2, 10496 CrossRef CAS; (d) R.-G. Shi, J. Sun and C.-G. Yan, ACS Omega, 2017, 2, 7820 CrossRef CAS.
- CCDC 1911986 (Cambridge Crystallographic Data Centre, https://www.ccdc.cam.ac.uk) contains the supplementary crystallographic data for 5b.†.
- For two recent asymmetric PTC contributions by our group: (a) J. Novacek, J. A. Izzo, M. J. Vetticatt and M. Waser, Chem. – Eur. J., 2016, 22, 17339 CrossRef CAS PubMed; (b) V. Capaccio, K. Zielke, A. Eitzinger, A. Massa, L. Palombi, K. Faust and M. Waser, Org. Chem. Front., 2018, 5, 3336 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1911986. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ob01134j |
| This journal is © The Royal Society of Chemistry 2019 |