Dehydrogenative C(sp3)–H bond functionalization of tetrahydroisoquinolines mediated by organic oxidants under mild conditions†
Abstract
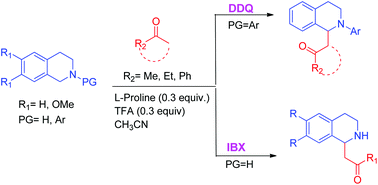
The organocatalyzed Mannich reaction of unsubstituted and N-aryl-substituted tetrahydroisoquinolines (THIQs) and the Strecker reaction of several N-aryl-substituted THIQs through dehydrogenative C(sp3)–H bond functionalization (cross-dehydrogenative coupling) promoted by organic single electron oxidants DDQ and IBX are presented. The C–H oxidation/Mannich reaction of less reactive N-aryl substituted pyrrolidines is achieved via metal catalyzed photoredox catalysis. Operationally simple procedures provide desired products in an effective and time preserving manner.
- This article is part of the themed collections: Synthetic methodology in OBC and Direct C-H Functionalization in Method Development and Late Stage Functionalization


 Please wait while we load your content...
Please wait while we load your content...