One stone two birds: cobalt-catalyzed in situ generation of isocyanates and benzyl alcohols for the synthesis of N-aryl carbamates†
Abstract
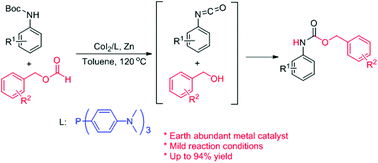
An efficient method for the synthesis of N-aryl carbamates from N-Boc-protected amines has been developed. The cobalt-catalyzed in situ generation of isocyanates from N-Boc-protected amines and benzyl alcohols from benzyl formates has been achieved for the first time, which in turn furnished the corresponding benzyl carbamates in moderate to high yields. The reaction was catalyzed by CoI2 with tris-(4-dimethylaminophenyl)-phosphine as the ligand and zinc powder as the reductant. The developed reaction conditions were found to be compatible for aromatic amines with both electron-donating and -withdrawing substituents.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...