Selective synthesis of 3-deoxy-5-hydroxy-1-amino-carbasugars as potential α-glucosidase inhibitors†
Abstract
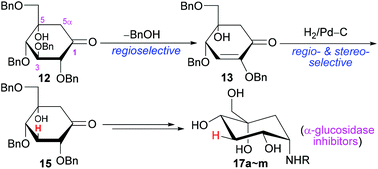
A convenient synthesis of novel 3-deoxy-5-hydroxy-1-aminocarbasugars was developed here. The benzyl-protected glucose-derived ketone 12 was selectively converted in high yield to enone 13via retro-Michael elimination of BnOH. The double bond of 13 was regio- and stereo-selectively reduced by the induction of C4-α-OBn to the multi-functionalized 15. 15 contained all the functionalities with similar configurations to carbasugars but with 3-H and 5-OH in the ring, and it would be a very interesting building block for organic synthesis or for bioactive compounds. As one application, 15 was further transformed into 1-amino-carbasugars by the reductive amination and final deprotection of benzyls. The targets were subjected to the in vitro inhibitory activity test against sucrase or maltase. The inhibitory activity of 17b, 17h or 17j against sucrase was nearly similar to that of voglibose. In comparison with voglibose, in vivo results similarly showed that 17b, 17h or 17j could lower the post-prandial blood glucose level after sucrose loading in healthy male ICR mice, while miglitol or acarbose was less effective. The molecular modeling study of some targets or voglibose with human sucrase could explain the inhibiting action.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...