Covalently linked meso-tetraaryl triphyrin(2.1.1)-ferrocene(s) conjugates: synthesis and properties†‡
Abstract
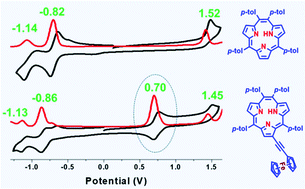
Functionalized meso-tetraaryl triphyrins(2.1.1) containing two meso-iodophenyl groups or a bromo group at the β-pyrrole carbon were synthesized over a sequence of steps. The covalently linked conjugates such as a triphyrin(2.1.1)-(ferrocene)2 triad and a triphyrin(2.1.1)-ferrocene dyad were obtained in 47–51% yields by coupling the appropriate functionalized triphyrin with ethynylferrocene under mild Pd(0) coupling reaction conditions. The functionalized triphyrins(2.1.1) and their conjugates were thoroughly characterized by HR-mass spectrometry and 1D and 2D NMR spectroscopy. The absorption, electrochemical and DFT studies suggested weak interactions between the triphyrin(2.1.1) and ferrocenyl moieties in triads but relatively strong interactions in dyads.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...