An unprecedented intramolecular to intermolecular mechanistic switch in 1,1-diaminoazines leading to differential product formation during the I2-induced tandem oxidative transformation†
Abstract
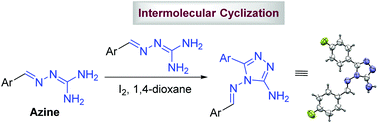
The tautomeric preference of guanylhydrazones towards the azine form induces an unprecedented intramolecular to intermolecular mechanistic switch during the I2-catalyzed oxidative transformation leading to 4,5-disubstituted-3-amino-1,2,4-triazoles in contrast to the reaction of semicarbazones and thiosemicarbazones to form 1,3,4-oxa/thiadiazoles. This intramolecular to intermolecular cyclization shift was established through control experiments and was attributed to the high energy demand (∼22 kcal mol−1) for the azine tautomer to adopt the s-cis conformation which is essential for the intramolecular reaction. An I2 induced protocol for an efficient and straightforward synthesis of 4,5-disubstituted-3-amino-1,2,4-triazoles has been developed via tandem oxidative transformation of guanylhydrazones (in its preferentially existing azine tautomeric form) with distinct advantages such as wide substrate scope, use of substoichiometric amounts of iodine, no requirement of external oxidizing agents, base free reaction conditions, short reaction time and moderate to good yields. The role of silver salt in improving the yield and shortening of reaction time was also highlighted.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...